Polypeptide-manganese-carbonyl compound CO releasing molecule taking phenylazo pyridine as ligand as well as synthesis method and application of polypeptide-manganese-carbonyl compound CO releasing molecule
A technology of phenylazopyridine and complexes, which is applied in the field of synthesis and preparation of polypeptide-metal complexes, can solve the problems of low yield, achieve the effect of simplifying the number of synthesis steps and shortening the time required for synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
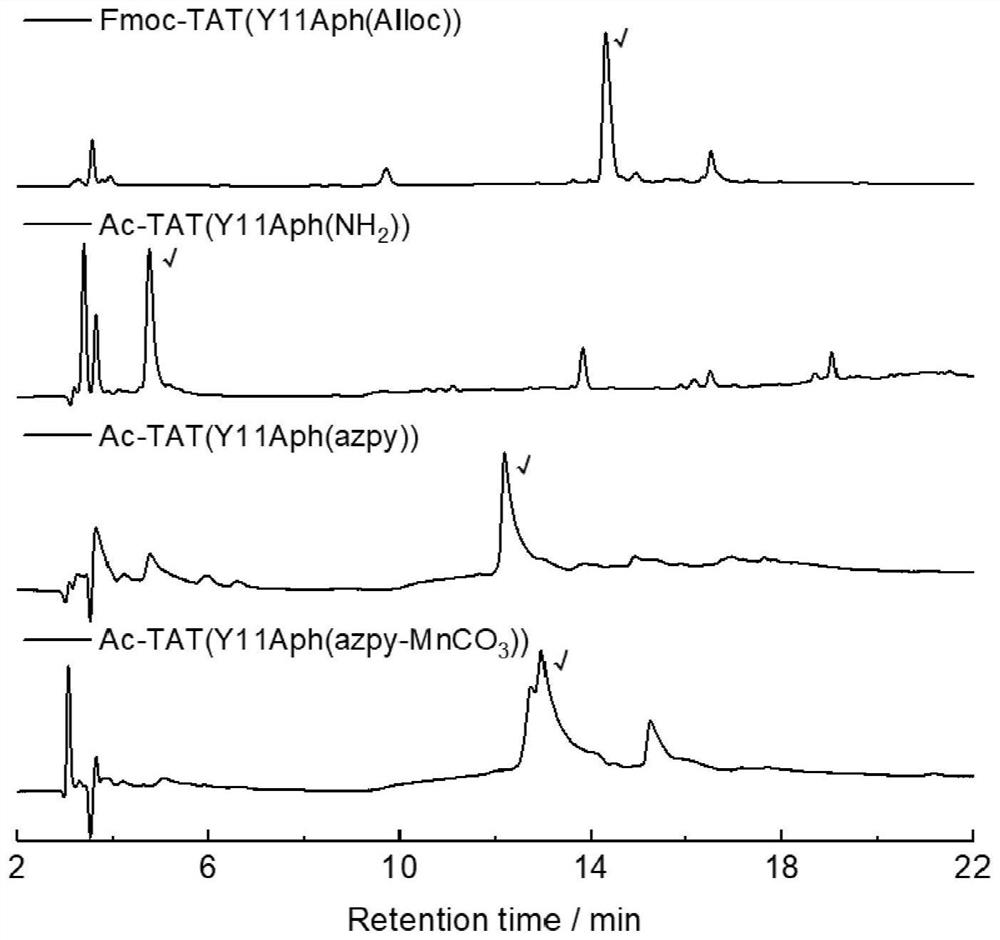
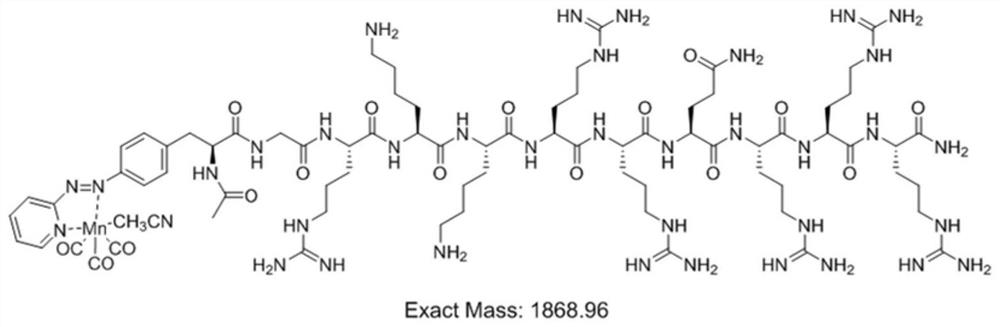
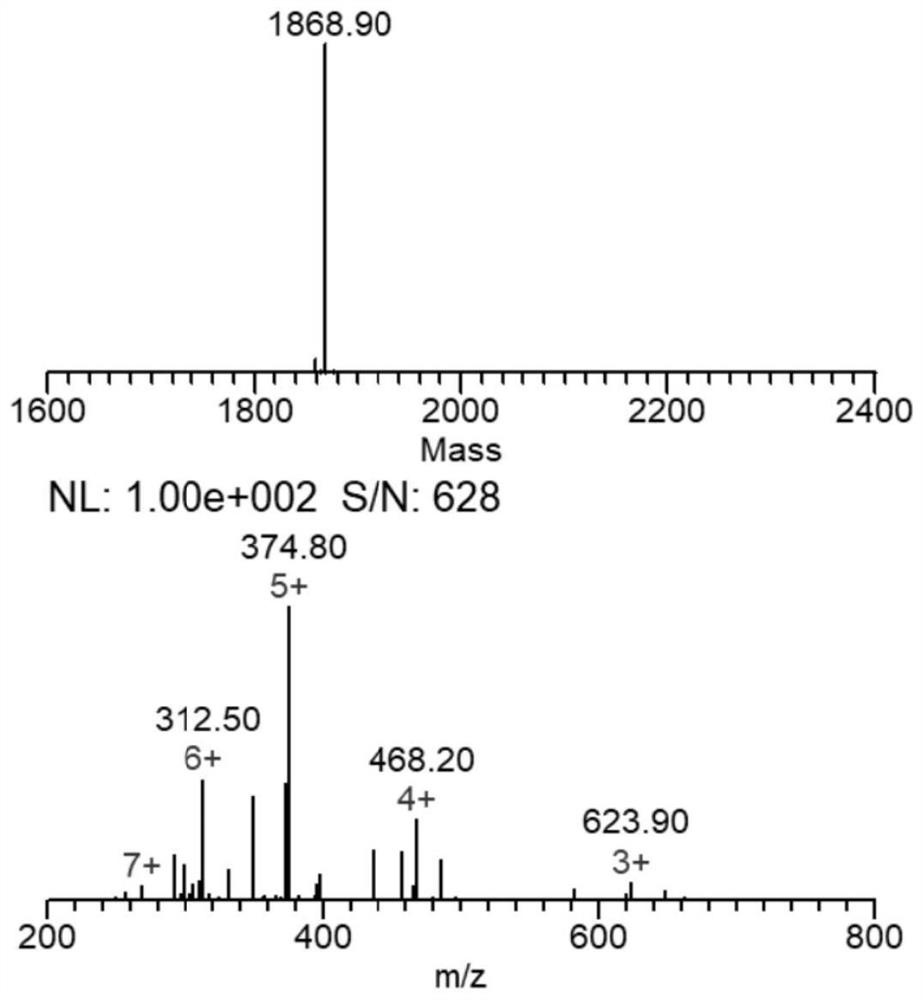
[0085] Embodiment 1: The preparation method of the TAT-manganese carbonyl complex whose side chain coordinating group is phenylazopyridine (azpy):
[0086] (1) Preparation of Fmoc-Arg(Pbf)-MBHA resin: Take 500mg Rink Amide MBHA resin in a peptide synthesis tube with a loading capacity of 0.2-0.8mmol / g, add 15mL DMF to swell twice at room temperature, 15min each time, Drain, add 10mL 20% piperidine / DMF to the resin, shake at room temperature for 5min, wash twice with DMF, add 10mL 20%piperidine / DMF again, shake for 5min at room temperature, wash with DMF, DCM, DMF was washed twice each, and the solvent was drained to obtain the resin that the amino group removed Fmoc protection. Weighed Fmoc-Arg(Pbf)-OH(1mmol), TBTU(2mmol) and DIEA(0.98mmol), and Fmoc-Arg(Pbf )-OH and TBTU were dissolved with a small amount of DMF, added DIEA, and the carboxyl group was activated by shaking at room temperature for 2 minutes. After that, the activated amino acid was added to the resin, shaken an...
Embodiment 2
[0094] Example 2: TAT(Y11Aph(azpy)-Mn(CO) 3 ) light-controlled CO release experiment
[0095] Experimental material: TAT(Y11Aph(azpy)-Mn(CO) 3 ), PBS buffer, visible red light flashlight (~700nm, LED, 5W).
[0096] Experimental steps:
[0097] ① The PBS buffer was deoxygenated by bubbling nitrogen gas for 30 minutes.
[0098] ②Add TAT(Y11Aph(azpy)-Mn(CO) 3 ) polypeptide-manganese complex (final concentration is 0.16mg / mL).
[0099] ④Use the visible light and red light flashlight contrast cuvette for illumination, and use the ultraviolet-visible spectrum to record the spectrum of different cumulative illumination time. The result is as Figure 5 shown.
[0100] The following conclusions can be obtained from the changes of the characteristic peaks of the ultraviolet spectrum ① TAT-Mn(CO) without light 3 Will not release CO; ②After being illuminated by visible light and red light, TAT(Y11Aph(azpy)-Mn(CO) 3 ) in the metal-to-ligand electron transfer (MLCT) decreased, demo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com