A kind of solid-phase synthesis method and application of CO releasing molecule based on polypeptide-manganese-carbonyl complex
A solid-phase synthesis and complex technology, which is applied in the preparation method of peptides, chemical instruments and methods, peptides, etc., can solve the problems of lengthy and time-consuming synthesis process, complex and time-consuming preparation of SAAC, and shorten the synthesis time and cost , The effect of saving liquid phase purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
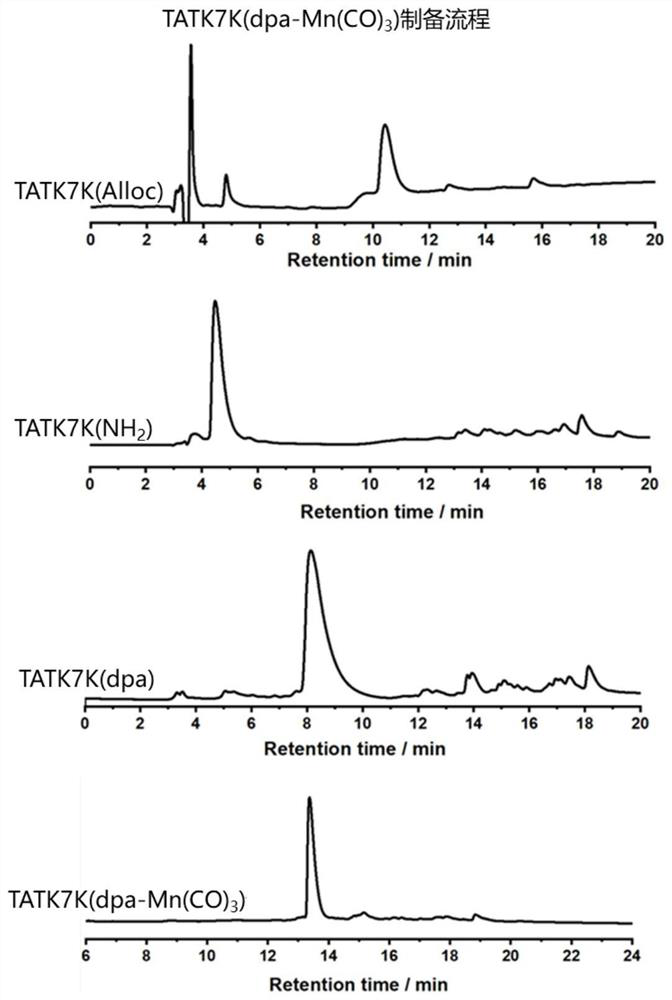
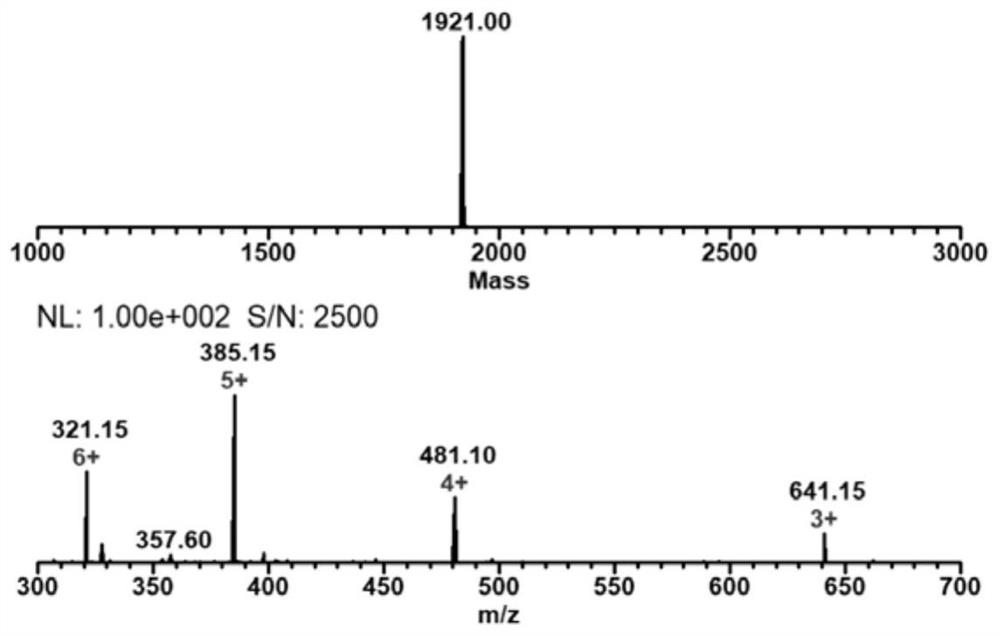
[0082] Example 1: The side chain coordinating group is the preparation method of bis-methylenepyridine (dpa) TAT-manganese carbonyl complex:
[0083](1) Preparation of Fmoc-Arg(Pbf)-MBHA resin: Take 500mg Rink Amide MBHA resin in a peptide synthesis tube with a loading capacity of 0.2-0.8mmol / g, add 15mL DMF to swell twice at room temperature, 15min each time, Drain, add 10mL 20% piperidine / DMF to the resin, shake at room temperature for 5min, wash twice with DMF, add 10mL 20%piperidine / DMF again, shake for 5min at room temperature, wash with DMF, DCM, DMF was washed twice each, and the solvent was drained to obtain the resin that the amino group removed Fmoc protection. Weighed Fmoc-Arg(Pbf)-OH(1mmol), TBTU(2mmol) and DIEA(0.98mmol), and Fmoc-Arg(Pbf )-OH and TBTU were dissolved with a small amount of DMF, added DIEA, and the carboxyl group was activated by shaking at room temperature for 2 minutes. After that, the activated amino acid was added to the resin, shaken and react...
Embodiment 2
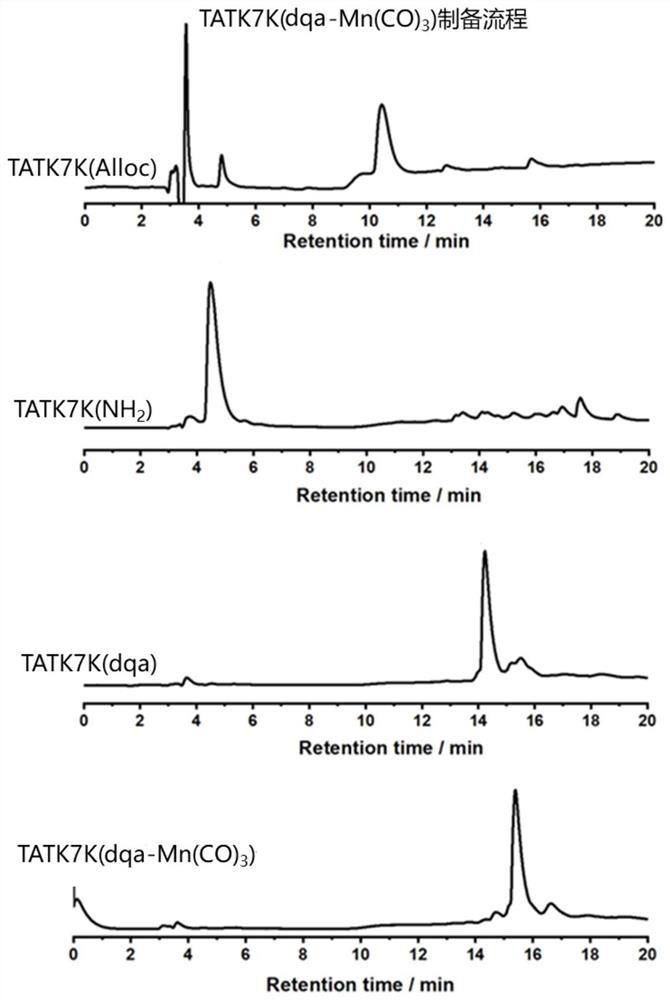
[0090] Embodiment 2: The side chain coordination group is the preparation method of double methylene quinoline (dqa) TAT-manganese carbonyl complex:
[0091] (1) According to the method of Example 1, 500 mg of Fmoc-Arg(Pbf)-MBHA resin with a loading capacity of about 0.44 mmol / g was obtained.
[0092] (2) Preparation of TATK7K(Alloc)-MBHA resin: add 15mL DMF to the obtained Fmoc-Arg(Pbf)-MBHA resin and swell twice at room temperature, 15min each time, drain, and then add 10mL 20% ethyl alcohol to the resin Acid anhydride / DMF, react with shaking at room temperature for 20min to block the amino group of the resin that is not coupled with amino acid, prevent its next reaction, wash twice with DMF, DCM, DMF in turn, add 10mL 20% piperidine / DMF to the resin, room temperature Shake the reaction for 5 minutes, wash twice with DMF, add 10 mL of 20% piperidine / DMF again, shake and react at room temperature for 5 minutes, wash twice with DMF, DCM, and DMF in turn, and drain the solvent ...
Embodiment 3
[0098] Example 3: Light-controlled CO release experiment
[0099] Experimental materials: myoglobin (Mb) (sigma aldrich), sodium dithionite, TATK7K (dpa-Mn(CO) 3 ), TATK7K (dqa-Mn(CO) 3 ), PBS buffer, visible violet flashlight (380-415nm, LED, 5W).
[0100] Experimental steps:
[0101] ① The PBS buffer was deoxygenated by bubbling nitrogen gas for 30 minutes.
[0102] ②Use deoxygenated PBS to prepare Mb to a final concentration of 40 μmol / L, and add to a quartz cuvette. Sodium dithionite (final concentration: 3.2mmol / L) was added to reduce Mb.
[0103] ③Add TAT-Mn(CO) 3 Polypeptide-manganese complex (final concentration: 10 μmol / L).
[0104]④Use the visible light purple light flashlight contrast color dish to illuminate, and use the ultraviolet-visible spectrum to record the spectrum of different cumulative illumination time. The result is as Figure 5 , Image 6 shown.
[0105] The following conclusions can be obtained from the changes of the characteristic peaks of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com