Live attenuated influenza vaccine composition and process for preparation thereof
A composition and influenza technology, applied in biochemical equipment and methods, medical preparations with non-active ingredients, polymer compounds with non-effective ingredients, etc., can solve problems such as no suitable chromatographic steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0224] Example 1: Stability data of rearranged LAIV virus immunogenic compositions
[0225] Component 1 - Live Attenuated Influenza Vaccine Virus (LAIV)
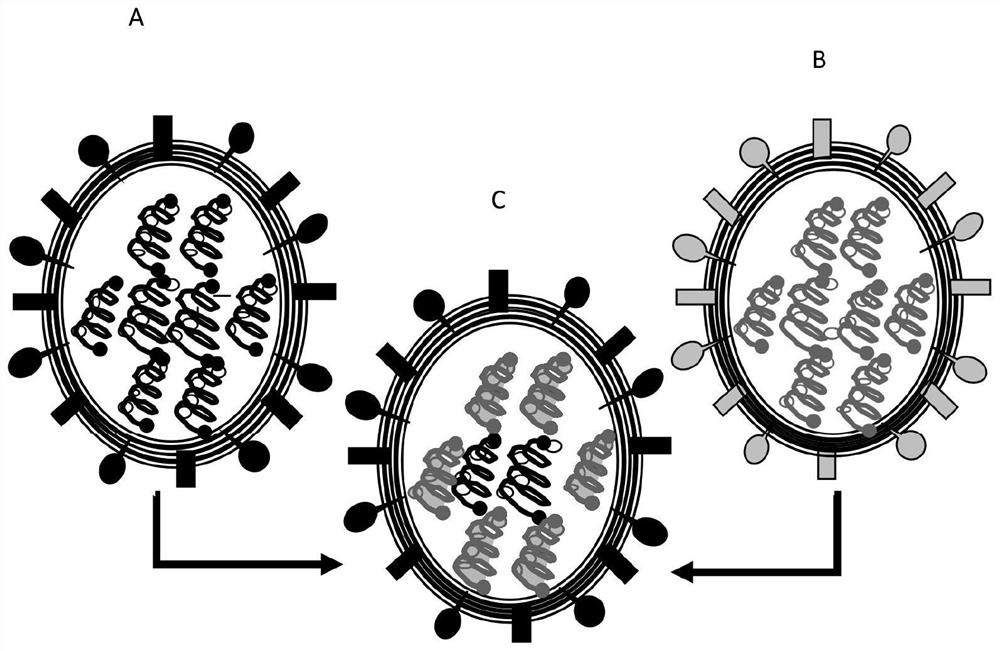
[0226] Influenza vaccine viruses are rearranged LAIV viruses derived by typical rearrangement methods that include the cold-adapted, temperature-sensitive and / or attenuated phenotypic genes of the master donor virus (MDV) in a 6:2 or 7:1 ratio Segments and hemagglutinin (HA) gene segments and / or neuraminidase (NA) gene segments of wild-type pandemic or seasonal influenza A or influenza B or influenza C strains.
[0227]
[0228]
[0229] For LAIV immunogenic compositions:
[0230] In the range of 6 to 7 log EID50 / 0.5 ml dose; more preferably 7 log EID50 / 0.5 ml dose of A virus
[0231] In the range of 6 to 7 log EID50 / 0.5 ml dose; more preferably 6.5 log EID50 / 0.5 ml dose of B virus
[0232] With respect to the rearranged LAIV strains used in the immunogenic compositions disclosed in Table 1 in any combination, the L...
Embodiment 2
[0246] Embodiment 2: LAIV virus manufacturing process based on MDCK cells
[0247] A method of preparing an MDCK cell culture-based LAIV composition may comprise any subset or all of the following steps:
[0248] k) Passage of LAIV candidate vaccine virus initially in SPF chicken embryos producing egg-based master seed virus (MSV).
[0249] 1) Egg-based master seed virus was grown on MDCK cell culture (ATCC CCL-34) hosts to make cell-based working seed virus (WSV). This cell-based WSV is used in different cell culture vessels / systems (e.g. with a surface area of 175 cm 2 tissue culture flasks (TCFs) with a surface area of 850 cm 2 roller bottles (RBs) with a surface area of 6320cm 2 Cell factories (CFs) and fixed-bed bioreactors (e.g., from Life Sciences Inc. (Port Washington, New York) Infect MDCK cell cultures at an MOI of 1:100 to 1:10000 in bioreactors such as Nano bioreactor and 500 / 100 bioreactor)). (MDCK cells were cultured in MEM containing FBS; rinsed wi...
Embodiment 3
[0270] Example 3: Effect of virus input (MOI) and post-inoculation incubation period on yield
[0271]
[0272]
[0273] Infect:
[0274] A. Infection time:
[0275] Based on optimization studies, the upper limit of the number of cells infected with MDCK cell-derived influenza working seed virus was selected to be 120 to 180 million cells per roller bottle and 7 to 10 billion cells per bioreactor system. Microscopic observation of monolayer confluence was performed on roller bottles with MDCK cells prior to the infection procedure.
[0276] B. MOI and post-inoculation incubation period:
[0277] Based on all observations from MOI optimization studies, MOIs for influenza A(H1N1), A(H3N2) and B viruses were chosen to range from 1:100 to 1:10000, and post-inoculation incubation periods were between 48 and 72 hours between.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com