Preparation method of high-conductivity perovskite type BaZrO3-based proton conductor material with controllable grain size
A high-conductivity, perovskite-type technology, applied in chemical instruments and methods, circuits, fuel cells, etc., can solve the problems of difficult grain growth, unsuitable for BaZrO application, uncontrollable grain size, etc. The effects of reduced boundary impedance, improved total conductivity, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
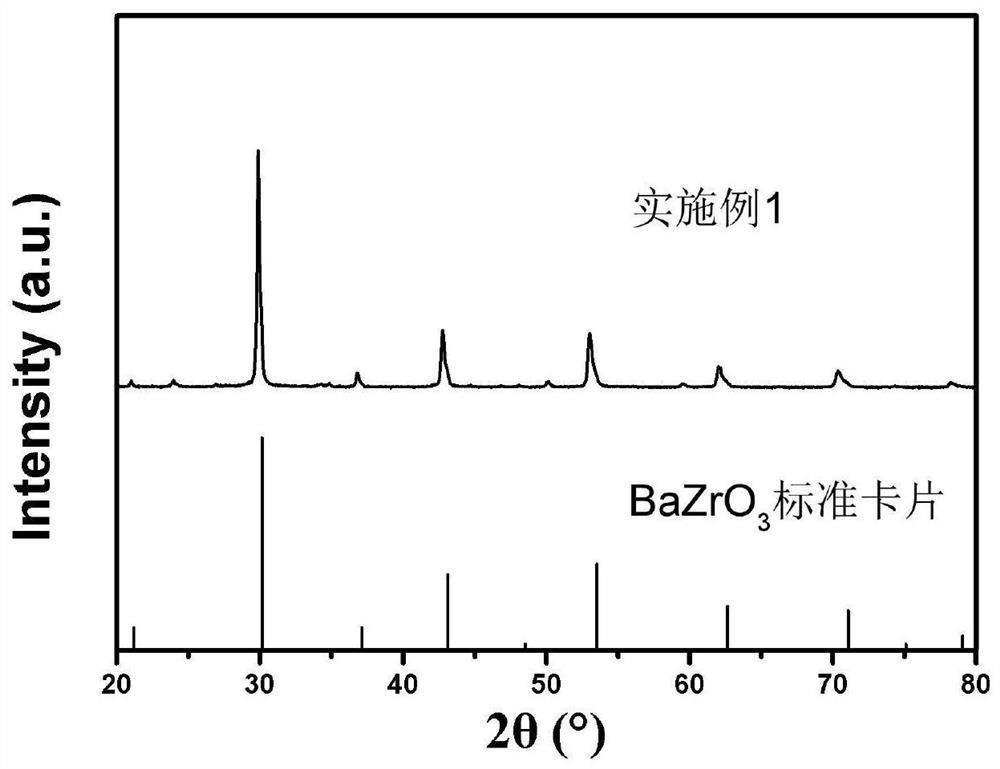
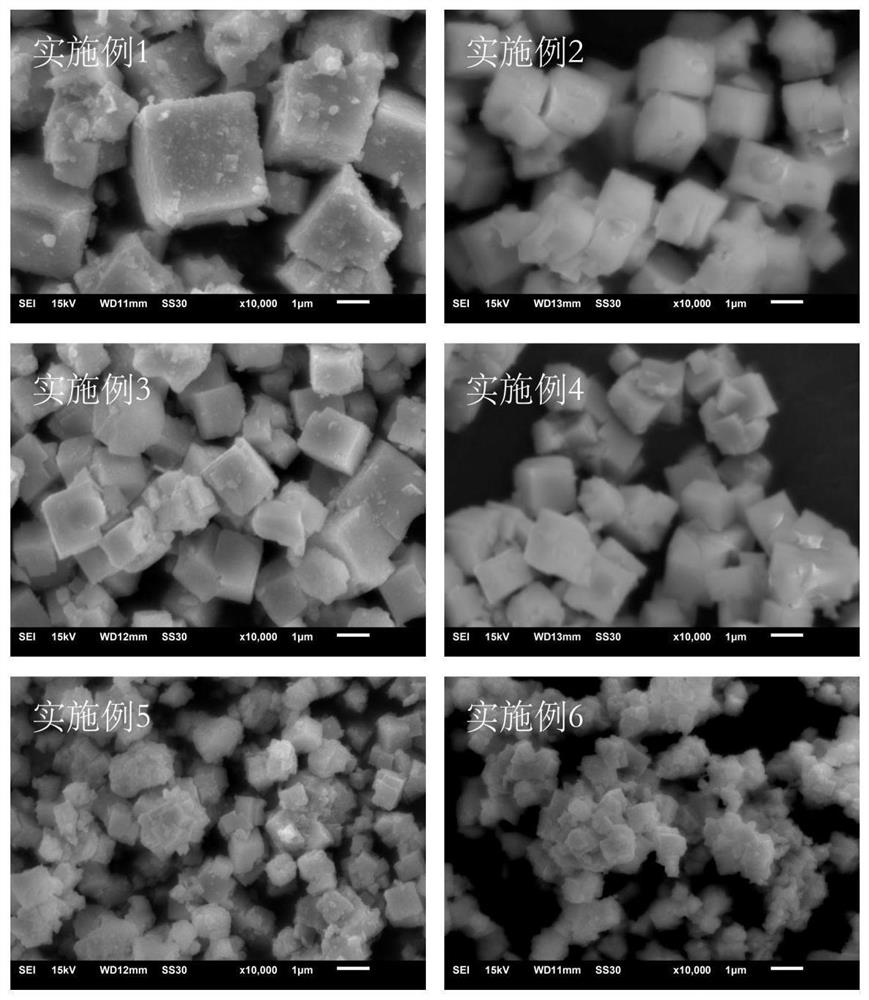
[0028] Weigh 1 mole of barium nitrate, add it to an appropriate amount of deionized water, and after the barium nitrate is completely dissolved, add 1 mole of (ZrO 2 ) 0.92 (Y 2 O3) 0.08 (particle size is 0.15-5 μm), placed on a magnetic stirrer, heated at 80° C. and continuously stirred to volatilize water to obtain dry mixed powder. Then the mixed powder was placed in an alumina crucible and placed in a tubular muffle furnace for calcination. During the heating process, the heating rate was 1.33°C / min, and finally kept at 800°C for 2 hours. Nitrogen was introduced into the tubular muffle furnace during the reaction, and the flow rate of nitrogen was 60 mL / min. After cooling, the XRD pattern of the synthesized BZY15 powder is as follows figure 1 shown. It can be seen from the figure that the characteristic diffraction peaks of the samples are all consistent with those of BaZrO 3 The standard card corresponds to the successful synthesis of BZY15 powder. The grain size ...
Embodiment 2
[0030] Weigh 1 mole of barium nitrate, add it to an appropriate amount of deionized water, and after the barium nitrate is completely dissolved, add 1 mole of (ZrO 2 ) 0.92 (Y 2 O3) 0.08 (particle size is 0.15-5 μm), placed on a magnetic stirrer, heated at 80° C. and continuously stirred to volatilize water to obtain dry mixed powder. Then the mixed powder was placed in an alumina crucible and placed in a tubular muffle furnace for calcination at a heating rate of 2.22°C / min, and finally kept at 800°C for 3h. Nitrogen was fed into the tubular muffle furnace during the reaction, and the flow rate of nitrogen was 60 mL / min. After cooling, the grain size of the synthesized powder is about 1.428μm. The electrochemical test steps are the same as in Example 1. At 600°C, its conductivity reaches 4.44×10 -3 S cm -1 .
Embodiment 3
[0032]Weigh 1 mole of barium nitrate, add it to an appropriate amount of deionized water, and after the barium nitrate is completely dissolved, add 1 mole of (ZrO 2 ) 0.92 (Y 2 O3) 0.08 (particle size is 0.15-5 μm), placed on a magnetic stirrer, heated at 80° C. and continuously stirred to volatilize water to obtain dry mixed powder. Then the mixed powder was placed in an alumina crucible and placed in a tubular muffle furnace for calcination. During the heating process, the heating rate was 3.33°C / min, and finally kept at 850°C for 2h. Nitrogen was passed through during the reaction, and the flow rate of nitrogen was 20 mL / min. After cooling, the grain size of the synthesized powder is about 1.337μm. The electrochemical test steps are the same as in Example 1. At 600°C, its conductivity reaches 4.32×10 -3 S cm -1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com