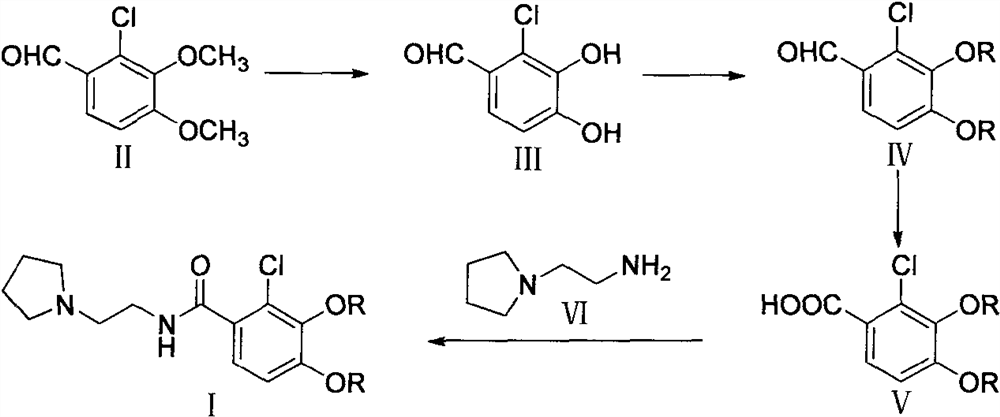
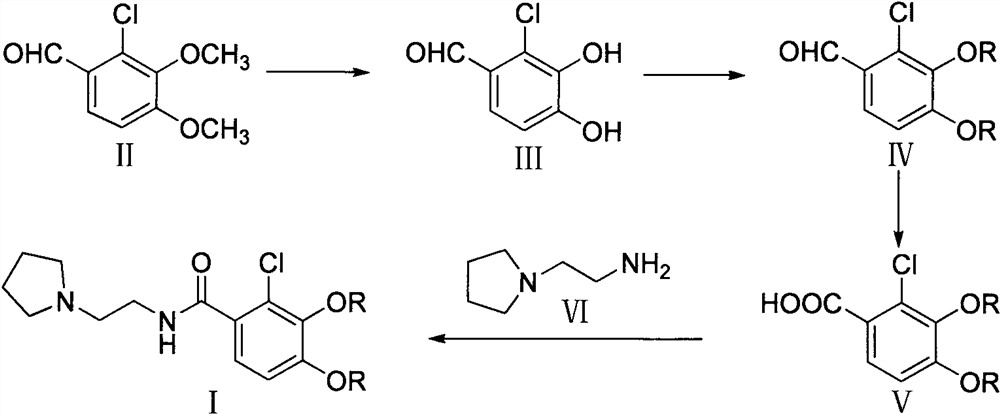
Preparation method of cefiderocol side chain
A side chain, cephalosporin technology, applied in the field of drug synthesis, can solve the problems of large environmental pollution, high synthesis cost, difficult operation, etc., and achieves the effects of easy industrial production, safe industrial conditions, and simple reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Step 1, the preparation of 2-chloro-3,4-dihydroxybenzaldehyde
[0035] Under nitrogen protection, add 10.0g (49.85mmol) 2-chloro-3,4-dimethoxybenzaldehyde and 50mL CH 2 Cl 2 , add 14.0g (104.99mmol) of anhydrous AlCl under stirring at room temperature 3 , and then dropwise added 33.8mL (419.98 mmol) of pyridine, after dropping, refluxed for 8.0h. Add 200 mL of dilute HCl solution, stir, and a solid precipitates out. Cool for 1.0 h, filter, wash with water 3 times, suck dry, and dry under reduced pressure at 50°C to obtain 7.8 g of off-white solid III with a yield of 90.6%.
[0036] 1 H NMR (600MHz, DMSO-d 6 )δ10.92(s, 1H, OH), 10.07(s, 1H, CHO), 9.66(s, 1H, OH), 7.33(d, J=51.0Hz, 1H, ArH), 6.83(d, J= 15.1Hz, 1H, ArH).
[0037] 13 C-NMR (150MHz, DMSO-d 6 )δ189.21, 153.25, 142.68, 125.19, 124.36, 122.28, 114.04.
[0038] Step 2, the preparation of 2-chloro-3,4-bis((4-methoxybenzyl)oxy)benzaldehyde
[0039] Under nitrogen protection, add 10.0g (57.95mmol) 2-chlo...
Embodiment 2
[0051] Step 1, the preparation of 2-chloro-3,4-dihydroxybenzaldehyde
[0052] Under nitrogen protection, 10.0g (49.85mmol) 2-chloro-3,4-dimethoxybenzaldehyde and 50mL N, N-dimethylacetamide were added dropwise to a 250mL three-necked flask, and 18.6g ( 139.57mmol) AlCl 3 anisole (56 mL). Then 11.2 mL (139.57 mmol) of pyridine was added dropwise, heated to 55° C., and reacted for 5.0 h. Add 100mL dilute HCl solution and 100mL tetrahydrofuran, concentrate under reduced pressure, a large amount of solid precipitates, cool and stir for 2.0h, filter and wash with water 3 times. After drying under reduced pressure at 50°C, 8.1 g of off-white solid III was obtained, with a yield of 94.2%.
[0053] 1 H NMR (600MHz, DMSO-d 6 )δ10.89(s, 1H, OH), 10.03(s, 1H, CHO), 9.62(s, 1H, OH), 7.30(d, J=51.1Hz, 1H, ArH), 6.83(d, J= 15.3Hz,1H,ArH).
[0054] 13 C NMR (150MHz, DMSO-d 6 )δ190.12, 153.65, 141.36, 125.40, 123.23, 122.19, 113.93.
[0055] Step 2, the preparation of 2-chloro-3,4-d...
Embodiment 3
[0068] Step 1, the preparation of 2-chloro-3,4-dihydroxybenzaldehyde
[0069] Under nitrogen protection, add 10.0g (49.85mmol) 2-chloro-3,4-dimethoxybenzaldehyde and 60mLCH 2 Cl 2 , cooled to 0 ° C, slowly dropwise added 50.0g (199.38mmol) BBr 3 , warming up to 25°C for 3.0h. Concentrate under reduced pressure until no liquid flows out, and then add 100 mL of dilute HCl solution, and an off-white solid precipitates out. Cool and stir for 1.0h, filter, wash with water 3 times, and drain. After drying under reduced pressure at 50°C, 7.3 g of white solid III was obtained, with a yield of 84.9%.
[0070] 1 H NMR (600MHz, DMSO-d 6 )δ10.90(s, 1H, OH), 10.05(s, 1H, CHO), 9.68(s, 1H, OH), 7.32(d, J=51.0Hz, 1H, ArH), 6.83(d, J= 15.3Hz, 1H, ArH).
[0071] 13 C-NMR (150MHz, DMSO-d 6 )δ189.65, 153.33, 142.41, 126.38, 123.39, 122.11, 113.89.
[0072] Step 2, the preparation of 2-chloro-3,4-bis((4-methoxybenzyl)oxy)benzaldehyde
[0073] Under nitrogen protection, 10.0g (57.95mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com