Lithium-rich manganese-based material and application method thereof
A lithium-rich manganese-based, positive electrode material technology, applied in chemical instruments and methods, inorganic chemistry, nickel compounds, etc., can solve the problems of high manufacturing cost, short cycle life at high temperature, unstable performance of lithium batteries, etc., and achieve low manufacturing cost , stable performance, and the effect of overcoming poor high temperature characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
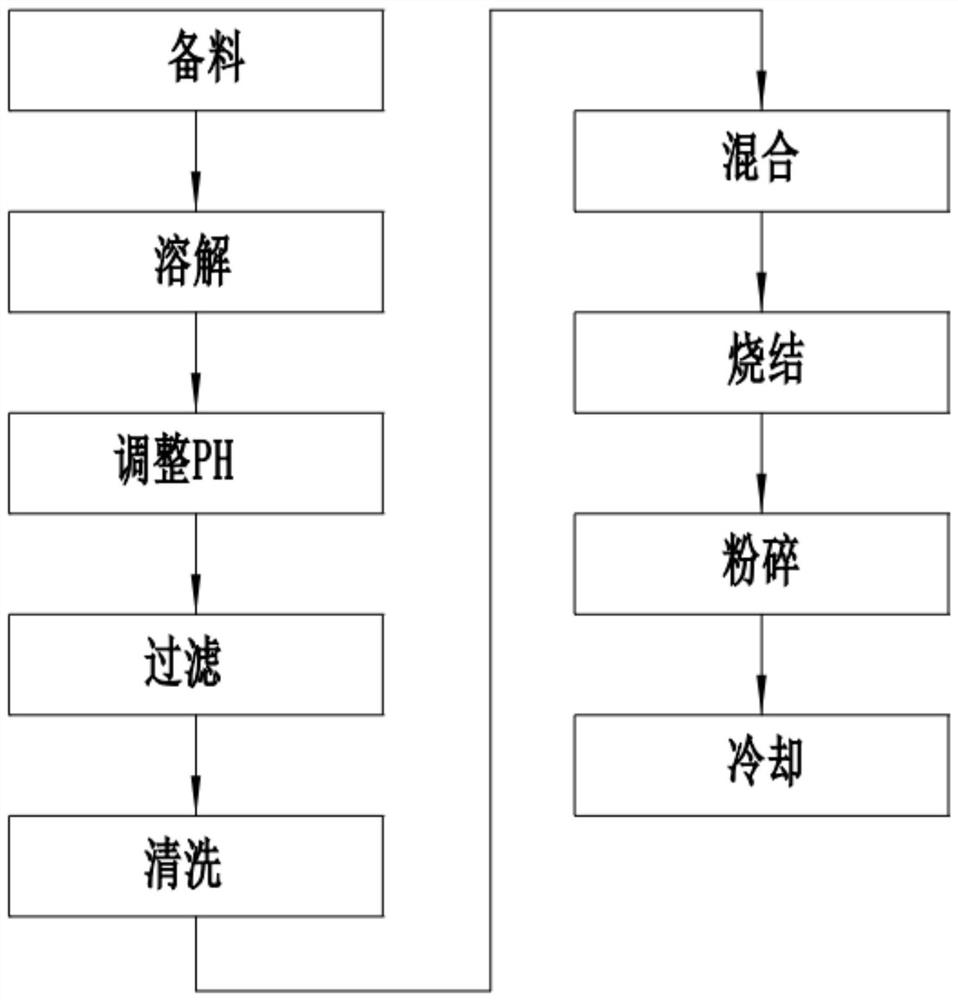
[0030] A lithium-rich manganese-based material, specifically comprising the following steps:
[0031] S1. material preparation: by weight, prepare some sulfates of nickel, manganese, cobalt, 50 parts of hydrochloric acid solution, 60 parts of ammoniacal liquor, 40 parts of sodium hydroxide solution;
[0032] S2. Dissolution: Take 50 parts of the hydrochloric acid solution in S1, dissolve the sulfates of nickel, manganese, and cobalt in the hydrochloric acid solution at a molar ratio of 0.35:0.6:0.05, and slowly add hydroxide Sodium solution, after the precipitation is generated, continue to add sodium hydroxide solution until the precipitate no longer increases, forming a solid-liquid mixture, preventing the waste of raw materials caused by adding too much sodium hydroxide solution;
[0033] S3. Adjust PH: slowly add ammonia water to the solid-liquid mixture in S2, and stir until the PH of the solid-liquid mixture is 8;
[0034] S4. Filtration: filter the solid-liquid mixture...
Embodiment 2
[0051] A lithium-rich manganese-based material, specifically comprising the following steps:
[0052] S1. material preparation: by weight, prepare some sulfates of nickel, manganese, cobalt, 50 parts of hydrochloric acid solution, 60 parts of ammoniacal liquor, 40 parts of sodium hydroxide solution;
[0053] S2. Dissolution: Take 50 parts of the hydrochloric acid solution in S1, dissolve the sulfates of nickel, manganese, and cobalt in the hydrochloric acid solution at a molar ratio of 0.35:0.6:0.05, and slowly add hydroxide Sodium solution, after the precipitation is generated, continue to add sodium hydroxide solution until the precipitate no longer increases, forming a solid-liquid mixture, preventing the waste of raw materials caused by adding too much sodium hydroxide solution;
[0054] S3. Adjust PH: slowly add ammonia water to the solid-liquid mixture in S2, and stir until the PH of the solid-liquid mixture is 8;
[0055] S4. Filtration: filter the solid-liquid mixture...
Embodiment 3
[0072] A lithium-rich manganese-based material, specifically comprising the following steps:
[0073] S1. get the raw materials ready: in parts by weight, prepare some vitriol salts of nickel, manganese, cobalt, 50 parts of hydrochloric acid solution, 60 parts of ammoniacal liquor, 40 parts of sodium hydroxide solution, the particle size of the vitriol salt of nickel, manganese, cobalt is uniform and without Impurities, the concentration of sodium hydroxide solution is 30%-50%;
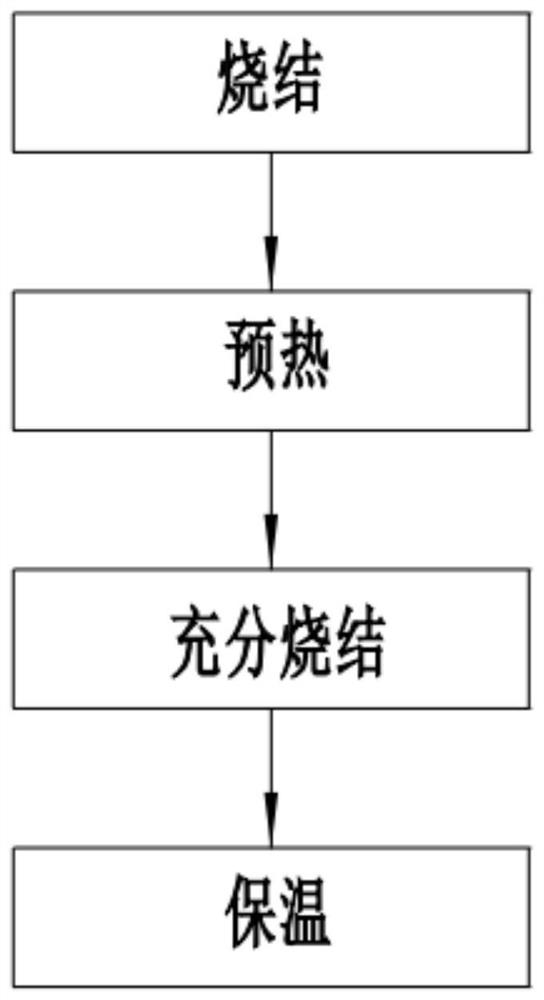
[0074] S2. Dissolution: Take 50 parts of the hydrochloric acid solution in S1, dissolve the sulfates of nickel, manganese, and cobalt in the hydrochloric acid solution at a molar ratio of 0.35:0.6:0.05, and slowly add hydroxide Sodium solution, after the precipitation is generated, continue to add sodium hydroxide solution until the precipitate no longer increases, forming a solid-liquid mixture, preventing the waste of raw materials caused by adding too much sodium hydroxide solution, the concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com