Preparation method and application of antituberculous drug Pretomanid
An anti-tuberculosis and drug technology, applied in the field of drug synthesis, can solve the problems of low yield, difficult removal, increased trouble, etc., and achieve the effects of high chemical and optical purity, clear impurity spectrum, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
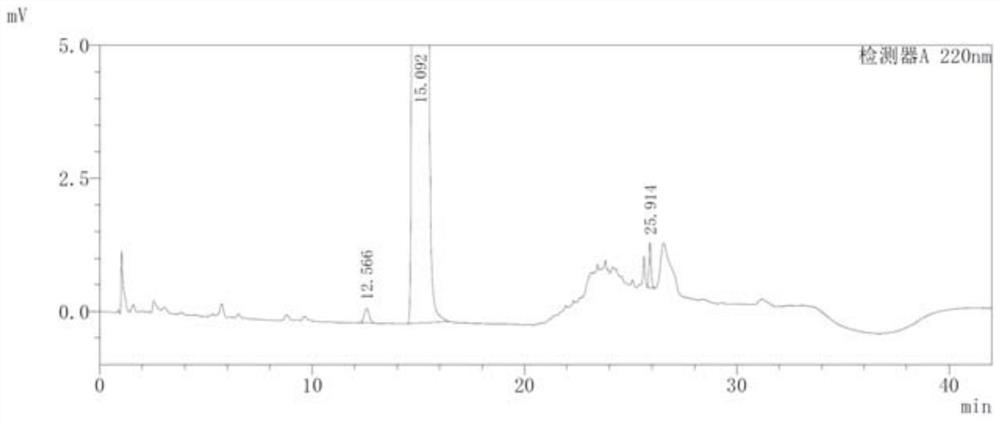
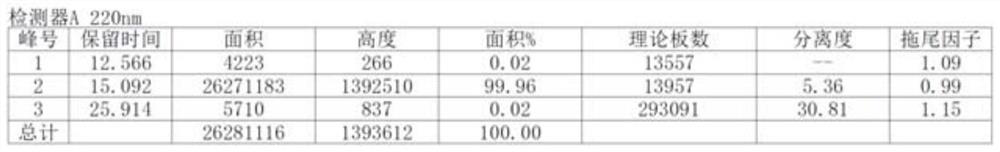
Image
Examples
Embodiment 1
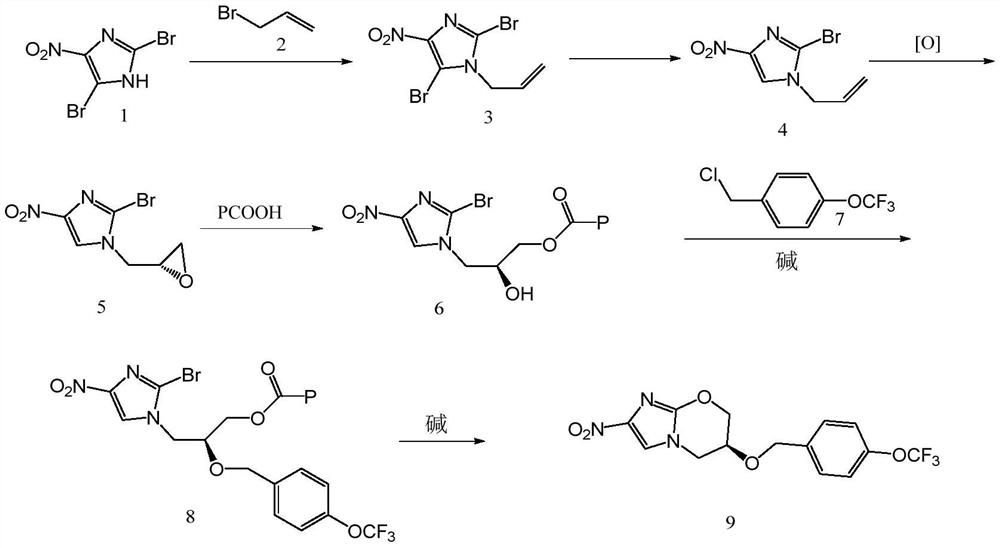
[0036] The preparation method of anti-tuberculosis drug Pretomanid comprises the steps:
[0037] Step 1: Preparation of 2,5-dibromo-4-nitro-1-(vinylmethyl)imidazole (compound 3)
[0038] 2,5-dibromo-4-nitroimidazole (compound 1) (351 g, 1.3 mol), 3-bromopropene (compound 2) (238.4 g, 1.97 mol) and potassium carbonate solid (539 g, 3.9 mol) were added In N,N-dimethylformamide (2500mL), react at 50°C for 4h under the protection of nitrogen. After the reaction was completed, the temperature was lowered with an ice bath, and the reaction product was added into aqueous sodium bicarbonate solution (2000 mL), and extracted with ethyl acetate (4×2000 mL). The extract was washed with deionized water (2000 mL), concentrated under reduced pressure, and dried to obtain a pale yellow waxy solid 2,5-dibromo-4-nitro-1-(vinylmethyl)imidazole (compound 3), 370.8 g, yield 92%.
[0039] Step 2: Preparation of 2-bromo-4-nitro-1-(vinylmethyl)imidazole (compound 4)
[0040] Na 2 SO 3 (228g, 1...
Embodiment 2
[0052] The preparation method of anti-tuberculosis drug Pretomanid comprises the steps:
[0053] Step 1: Preparation of 2,5-dibromo-4-nitro-1-(vinylmethyl)imidazole (compound 3)
[0054] 2,5-dibromo-4-nitroimidazole (compound 1) (351g, 1.3mol), 3-bromopropene (compound 2) (238.4g, 1.97mol) and sodium hydroxide solid (152g, 3.9mol) Add it into dimethyl sulfoxide (2500 mL), and react at 40° C. for 4 h under the protection of nitrogen. After the reaction was completed, the temperature was lowered with an ice bath, and the reaction product was added into aqueous sodium bicarbonate solution (2000 mL), and extracted with ethyl acetate (4×2000 mL). The extract was washed with deionized water (2000mL), concentrated under reduced pressure, and dried to obtain 2,5-dibromo-4-nitro-1-(vinylmethyl)imidazole (compound 3), a light yellow waxy solid, 363g , yield 90.1%.
[0055] Step 2: Preparation of 2-bromo-4-nitro-1-(vinylmethyl)imidazole (compound 4)
[0056] Na 2 S 2 o 3 (284.6g, ...
Embodiment 3
[0066] The preparation method of anti-tuberculosis drug Pretomanid comprises the steps:
[0067] Step 1: Preparation of 2,5-dibromo-4-nitro-1-(vinylmethyl)imidazole (compound 3)
[0068] 2,5-dibromo-4-nitroimidazole (compound 1) (351g, 1.3mol), 3-bromopropene (compound 2) (238.4g, 1.97mol) and 4-dimethylaminopyridine (476.5g, 3.9mol) was added to isopropyl acetate (2500mL), and reacted at 70°C for 4h under nitrogen protection. After the reaction was completed, the temperature was lowered with an ice bath, and the reaction product was added into aqueous sodium bicarbonate solution (2000 mL), and extracted with ethyl acetate (4×2000 mL). The extract was washed with deionized water (2000 mL), concentrated under reduced pressure, and dried to obtain a pale yellow waxy solid 2,5-dibromo-4-nitro-1-(vinylmethyl)imidazole (compound 3), 366.7 g, yield 91%.
[0069] Step 2: Preparation of 2-bromo-4-nitro-1-(vinylmethyl)imidazole (compound 4)
[0070] Na 2 S 2 o 5 (342g, 1.8mol) s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com