Application of MMI-0100 oligopeptide compound in preparation of medicine for treating cholestatic liver diseases
A technology for treating cholestasis and a drug, which is applied in the application field of MMI-0100 short peptide compound in the preparation of a drug for treating cholestatic liver disease, can solve problems such as obeticholic acid hepatotoxicity, and achieve the relief of the degree of bile duct hyperplasia and the expression of mRNA. The effect of reducing levels and reducing fibrotic lesions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
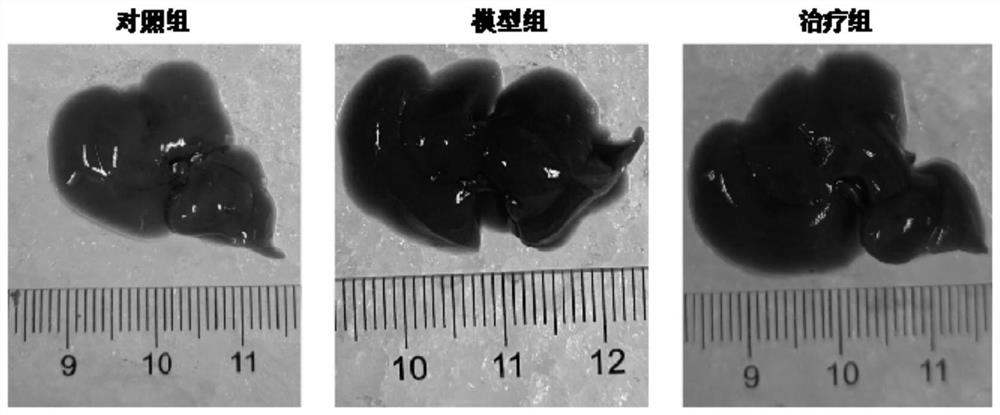
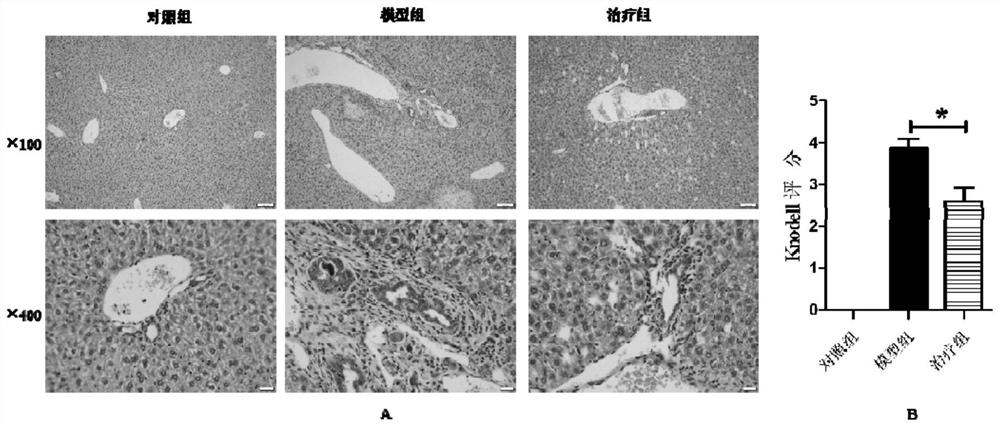
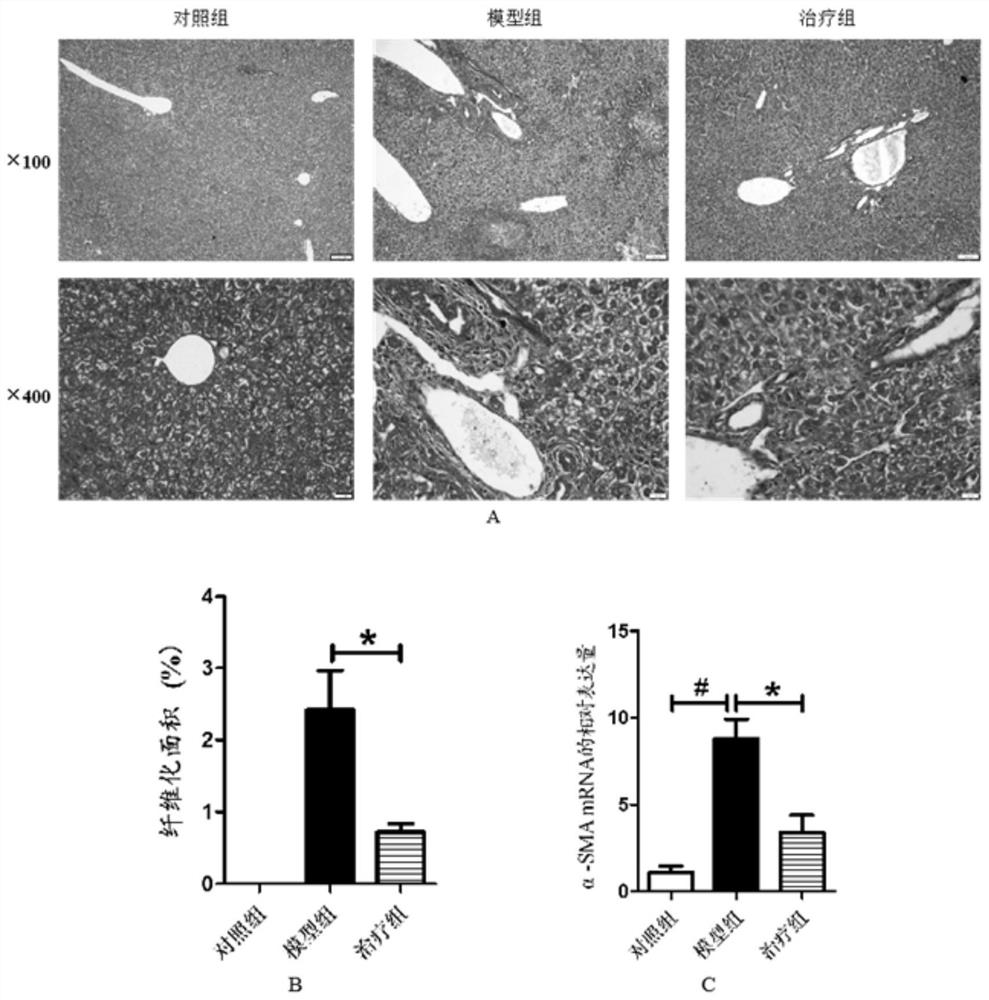
[0023] The histopathological changes of the liver
[0024] 1. Operation
[0025] Fifteen Balb / c mice were randomly divided into control group, model group (DDC) and treatment group (DDC+MMI-0100), with 5 mice in each group. Except for the control group, two groups of mice in the model group and the treatment group were fed 0.1% DDC irradiated feed to establish a cholestatic model. After 1w of DDC feeding, the mice in the treatment group were treated with MMI-0100 intraperitoneally every day. Mice were injected with a dose of 500ug / Kg, continuously injected for 1w, and given a normal diet at the same time. On the last day of drug injection, the mice were sacrificed by cervical dislocation and livers were collected.
[0026] Liver pathological detection: The intact mouse liver was isolated at low temperature, and the liver lesions of the mouse were observed with the naked eye. The tissue of about 5mm×5mm×2mm was taken, fixed in 10% paraformaldehyde for 24h, dehydrated in alcoh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com