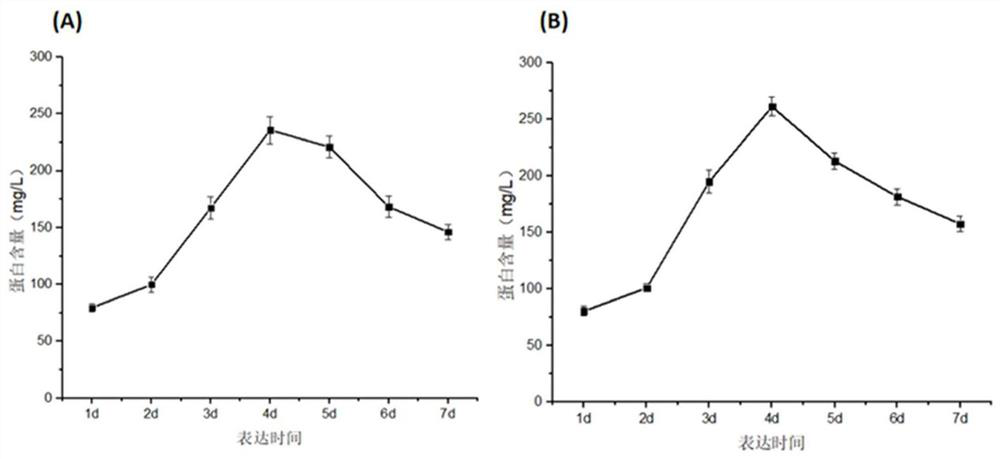
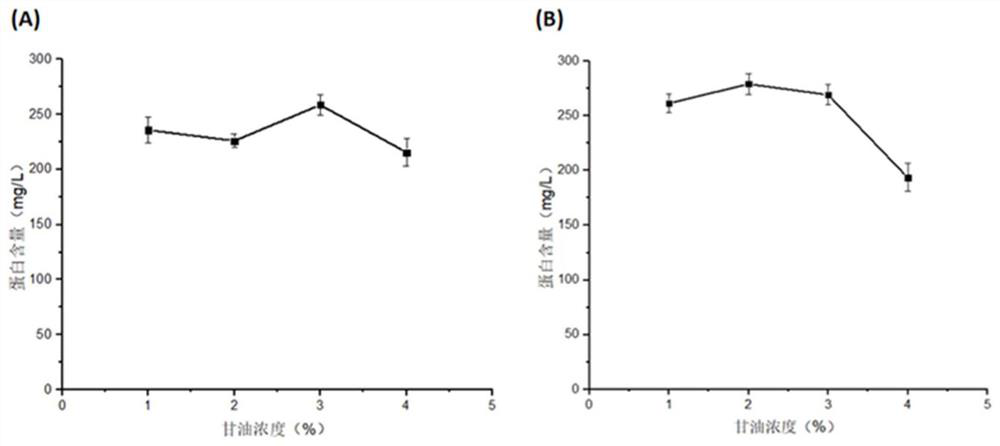
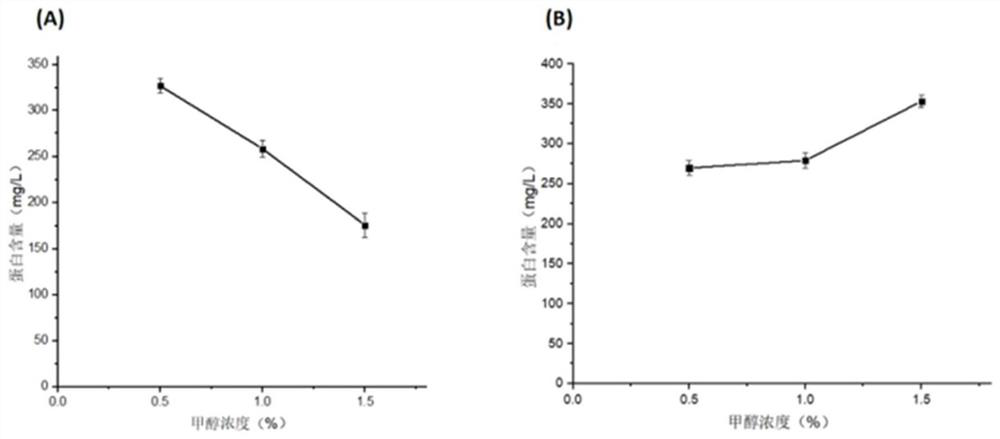
Application of protein in degradation of trypsin inhibitor
A technology of trypsin inhibition and protein, which is applied in the field of genetic engineering, can solve the problems of pancreatic hypertrophy, hyperplasia, and reduce the digestion and absorption of nutrients by organisms, and achieve the effect of efficient and stable degradation and increased expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This embodiment provides a method for preparing a protein with the ability to degrade trypsin inhibitors, including the following process:
[0040] 1. Codon optimization of peptidase S8 and peptidase M84 coding genes
[0041] First, use bioinformatics software to analyze the signal peptide regions of peptidase S8 and peptidase M84, and determine the amino acid sequences of the mature peptides of peptidase S8 and peptidase M84 with the C-terminal signal peptide removed, such as SEQ ID NO.1, SEQ ID NO As shown in .3, according to the codon preference of Pichia pastoris, in combination with codon optimization software and manual screening, peptidase S8 and peptidase through codon optimization as shown in SEQ ID NO.2, SEQ ID NO.4 are obtained The coding gene of M84 was subsequently synthesized.
[0042] 2. Construction of vectors expressing peptidase S8 and peptidase M84 and recombinant yeast
[0043] The coding genes (as shown in SEQ ID NO.2 and SEQ ID NO.4) of peptidase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com