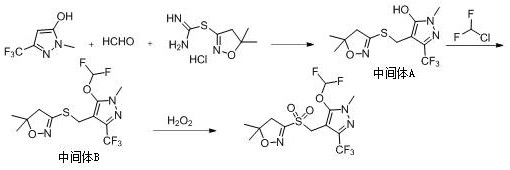
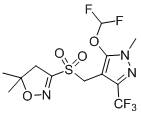
Preparation method of pyroxasulfone and intermediate thereof
A technology of sulfofenapyr and an intermediate, which is applied in the field of preparation of sulfofenapyr and its intermediates, can solve the problems of low yield of sulfofenazone, great harm to the environment and human beings, and teratogenicity of formaldehyde solvent. , to achieve the effect of high product purity, simplified production process and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Synthesis of Intermediate A:
[0041] Add 16.6 g of 1-methyl-3-(trifluoromethyl)-1H-pyrazol-5-ol, 3 g of paraformaldehyde, 8.5 g of piperidine, and 10 g of 37% hydrochloric acid aqueous solution in sequence in 40 mL of ethanol at room temperature, and heat Stir the reaction at 80°C for 4hrs, and add 5,5-dimethyl-3-mercapto-4,5 after no 1-methyl-3-(trifluoromethyl)-1H-pyrazol-5-ol is detected by HPLC -Dihydroisoxazole 21.6g, continue the reaction, continue to react for 4hrs, HPLC detects that the reaction of 5,5-dimethyl-3-mercapto-4,5-dihydroisoxazole is complete, after the reaction is completed, the ethanol is evaporated under reduced pressure , add 20mL of water to make a slurry for 2hr, suction filter to obtain a white solid precipitate, and dry to obtain 28.8g solid, which is Intermediate A. The purity measured by HPLC is 99.4%, and the yield is based on 1-methyl-3-(trifluoromethyl) -1H-pyrazol-5-ol was 92.6%.
[0042] 2. Synthesis of Intermediate B:
[0043] ...
Embodiment 2
[0047] 1. Synthesis of Intermediate A:
[0048] Add 33.2 g of 1-methyl-3-(trifluoromethyl)-1H-pyrazol-5-ol, 6 g of paraformaldehyde, 14.2 g of pyrrole, and 20 g of 37% hydrochloric acid aqueous solution in sequence in 80 mL of ethanol at room temperature, and heat to Stir the reaction at 75°C for 4 hours, and add 5,5-dimethyl-3-mercapto-4,5- Dihydroisoxazole 43.2g, continue to react, after continuing to react for 4hrs, HPLC detects that the reaction is over, the reaction solution is evaporated under reduced pressure to remove ethanol, 20mL of water is added to make a slurry for 2hr, and a white solid is precipitated by suction filtration, and dried to obtain 57.5g of solid, which is The purity of intermediate A measured by HPLC was 99.2%, and the yield was 92.3% based on 1-methyl-3-(trifluoromethyl)-1H-pyrazol-5-ol.
[0049] 2. Synthesis of Intermediate B:
[0050] Add 10.6g of sodium carbonate and 30.9g of intermediate A to 40mL of acetonitrile, stir at 20°C for 2hrs, slowl...
Embodiment 3
[0054] Synthesis of Intermediate A:
[0055] Add 33.2 g of 1-methyl-3-(trifluoromethyl)-1H-pyrazol-5-ol, 6 g of paraformaldehyde, 17.4 g of morpholine, and 20 g of 37% hydrochloric acid aqueous solution in sequence in 80 mL of ethanol at room temperature, and heat Stir the reaction at 60°C for 4hrs, and add 5,5-dimethyl-3-mercapto-4,5 after no 1-methyl-3-(trifluoromethyl)-1H-pyrazol-5-ol is detected by HPLC -Dihydroisoxazole 43.2g, continue to react, continue to react for 4hrs, HPLC detects that the reaction is over, evaporate ethanol under reduced pressure after the reaction, add 20mL water for beating for 2hrs, suction filter to obtain a white solid precipitate, and dry to obtain 56.8g solid, which is The purity of Intermediate A measured by HPLC was 99.3%, and the yield was 91.3% based on 1-methyl-3-(trifluoromethyl)-1H-pyrazol-5-ol.
[0056] 2. Synthesis of Intermediate B:
[0057] Add 10.6g of sodium carbonate and 30.9g of intermediate A to 40mL of acetonitrile, stir at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com