Preparation method of Filgotinib
A compound and alcohol solvent technology, which is applied in the field of preparation of Filgotinib, can solve the problems of cumbersome handling, unfriendly environment, and low product purity, and achieve the effects of improving product purity, simple post-treatment process, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
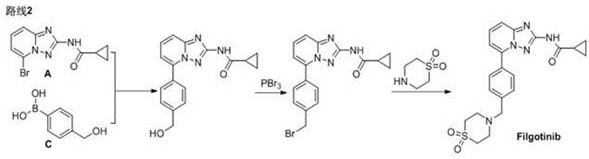
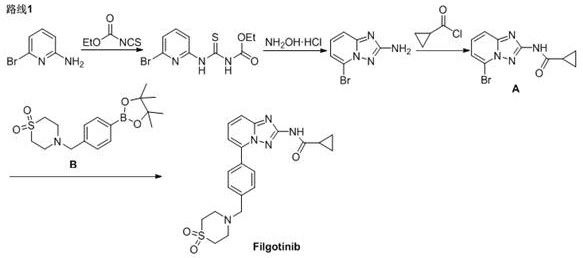
[0041] Compound A (75.0g, 0.27mol), compound B (98.4g, 0.28mol), NaHCO 3 (27.7g, 0.33mol), Pd(dppf)Cl 2 (2.0g, 2.7mmol), ethanol (600mL) and water (150mL) were added to the reaction flask, and after nitrogen replacement, the temperature was raised to 70-80°C for 15-20hrs, followed by HPLC until the reaction was complete.
[0042] Cool the reaction solution to 15~30°C and stir for 2~3hr. Suction filtration, the filter cake was rinsed with ethanol (150mL×2) and water to obtain the crude product of Filgotinib.
[0043]The above-mentioned Filgotinib crude product was added in the reaction flask, acetonitrile (4200mL) and water (600mL) were added, and heated to 65~80°C under stirring. o C to get a clear solution, add palladium removal reagent, keep warm for 60~70 o Stir at C, filter with suction, rinse the filter cake with acetonitrile (75mL×2), concentrate to 2250mL~3000mL, reduce to 0~10 o C stirring for 1~2hrs. Suction filtration, the filter cake was rinsed with acetonitril...
Embodiment 2
[0045] Compound A (7.50g, 27mmol), compound B (9.84g, 28mmol), K 2 CO 3 (4.55g, 33mmol), Pd(dppf)Cl 2 (0.2g, 0.27mmol), isopropanol (60mL) and water (6mL) were added to the reaction flask, and after nitrogen replacement, the temperature was raised to 80-90°C for 10-15hrs, followed by HPLC until the reaction was complete.
[0046] Cool the reaction solution to 15~30°C and stir for 2~3hr. Suction filtration, the filter cake was rinsed with ethanol (150mL×2) and water to obtain the crude product of Filgotinib.
[0047] The above-mentioned Filgotinib crude product was added in the reaction flask, acetonitrile (420mL) and water (60mL) were added, and heated to 65~80°C under stirring. o C to get a clear solution, add palladium removal reagent, keep warm for 60~70 o Stir at C, filter with suction, rinse the filter cake with acetonitrile (7.5mL×2), concentrate to 225mL~300mL, reduce to 0~10 o C stirring for 1~2hrs. Suction filtration, the filter cake was rinsed with acetonitrile...
Embodiment 3
[0049] Compound A (75.0g, 0.27mol), compound B (98.4g, 0.28mol), NaHCO 3 (27.7g, 0.33mol), Pd(dppf)Cl 2 (6.0g, 8.1mmol), ethanol (600mL) and water (150mL) were added to the reaction flask, and after nitrogen replacement, the temperature was raised to 70-80°C for 15-20 hrs, followed by HPLC until the reaction was complete.
[0050] Cool the reaction solution to 15~30°C and stir for 2~3hr. Suction filtration, the filter cake was rinsed with ethanol (150mL×2) and water to obtain the crude product of Filgotinib.
[0051] The above-mentioned Filgotinib crude product was added in the reaction flask, acetonitrile (4200mL) and water (600mL) were added, and heated to 65~80°C under stirring. o C to get a clear solution, add palladium removal reagent, keep warm for 60~70 o Stir at C, filter with suction, rinse the filter cake with acetonitrile (75mL×2), concentrate to 2250mL~3000mL, reduce to 0~10 o C stirring for 1~2hrs. Suction filtration, the filter cake was rinsed with acetonitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com