Preparation method of water-soluble polyporphyrin carrier-free nano-drug
A nano-drug, carrier-free technology, used in drug combinations, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of short circulation time, poor water solubility, and limited in vivo application, and achieve improved biocompatibility, Universal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the synthesis of water-soluble polyporphyrin P-3O
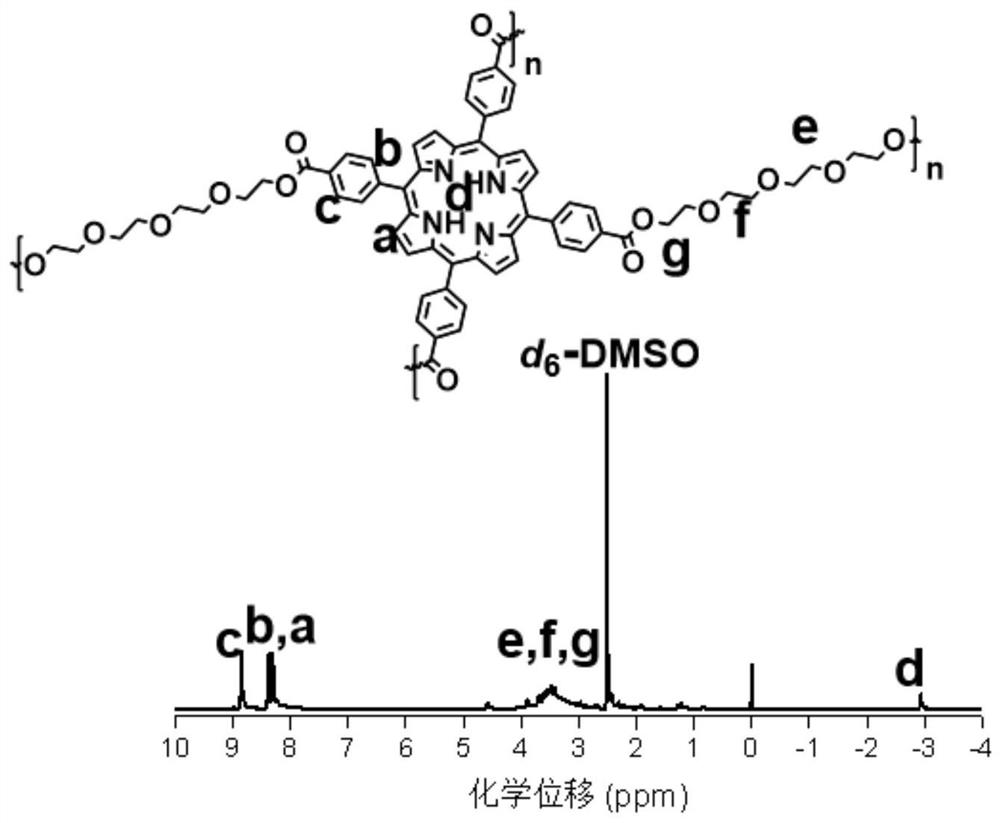
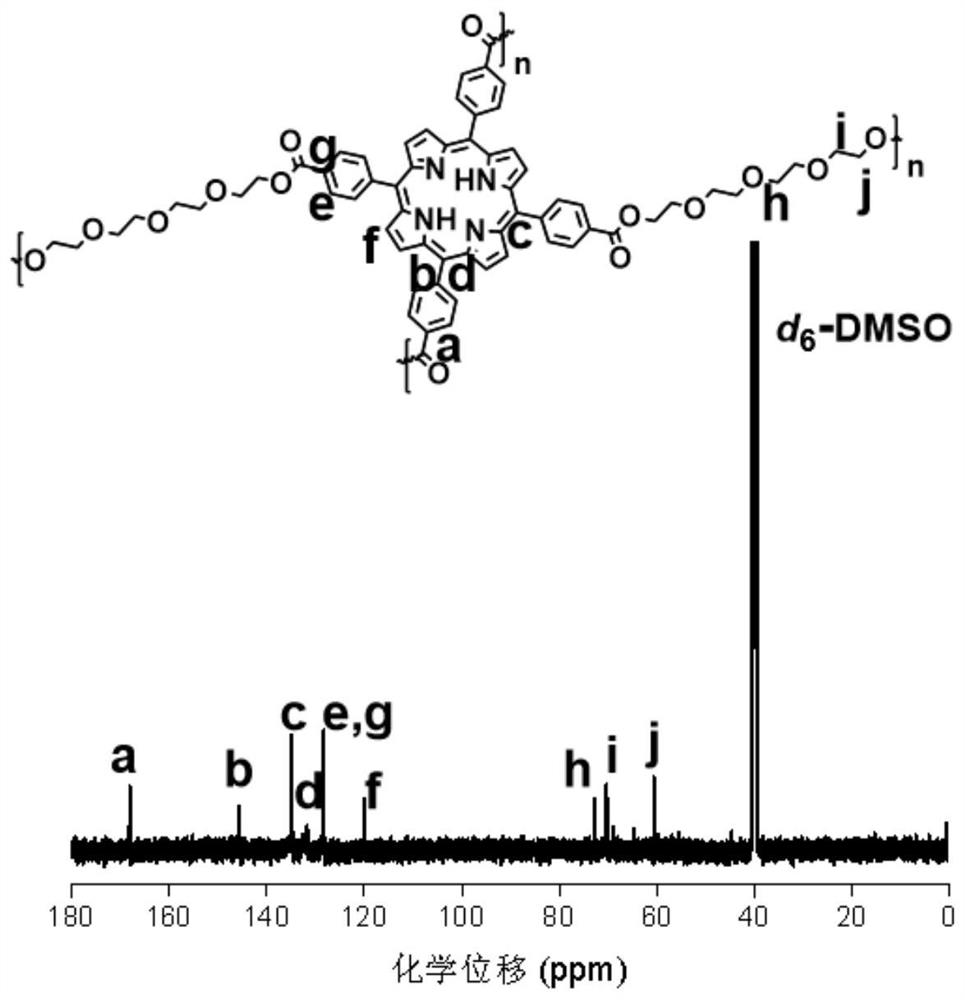
[0024] Accurately weigh tetracarboxyphenylporphyrin (25mg, 0.032mmol), EDCI (49mg, 0.256mmol) and DMAP (15.6mg, 0.128mmol) and dissolve in 0.2mL-2mL DMF, heat to 60°C-80°C and stir for 10min. Then, a mixed solution of tetraethylene glycol (12.4mg, 0.064mmol) and 0.1mL-0.5mL of DMF was added, and the temperature was raised to 100°C-140°C to continue the reaction for 24h-48h. After the reaction was completed, the reaction solution was settled in 15 mL of ether, the precipitate was collected by centrifugation, and the remaining ether was removed by vacuum drying overnight. Dissolve the precipitate in H at a volume ratio of 10:1 2 In a mixed solvent of O and DMSO, and dialyzed in deionized water for 24 h with a dialysis bag with a molecular weight cut-off (MWCO) of 500, and then freeze-dried in a freeze dryer, the final product was purple solid P-3O. The proton spectrum NMR characterization of gained polypo...
Embodiment 2
[0025] Embodiment 2: the synthesis of water-soluble polyporphyrin P-5O
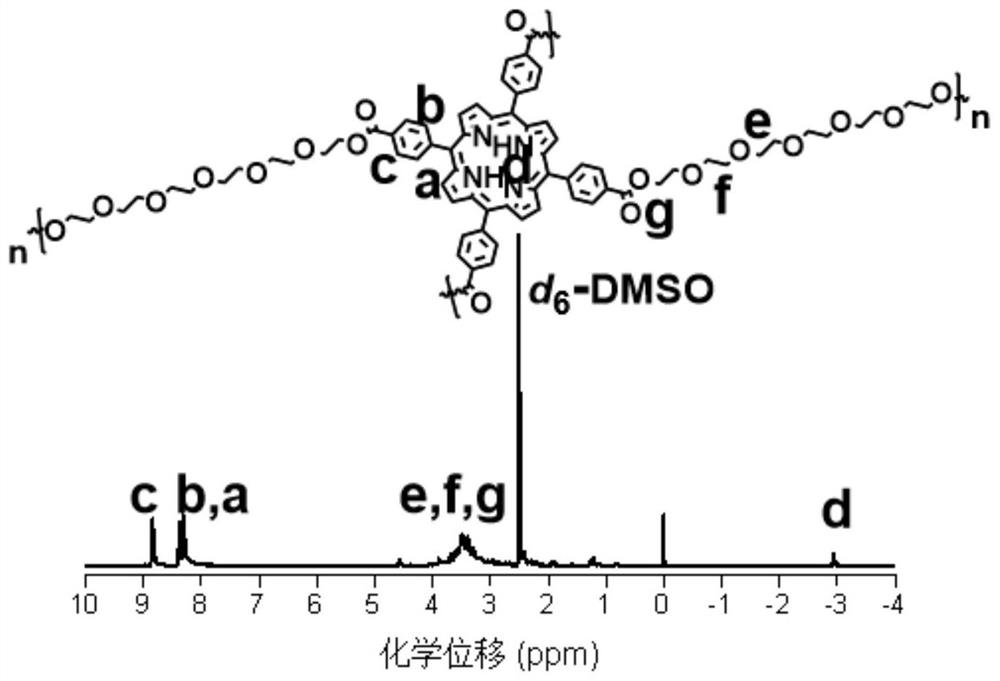
[0026] Accurately weigh tetracarboxyphenylporphyrin (25mg, 0.032mmol), EDCI (49mg, 0.256mmol) and DMAP (15.6mg, 0.128mmol) and dissolve in 0.2mL-2mL DMF, heat to 60°C-80°C and stir for 10min. Then, a mixed solution of hexaethylene glycol (17.6mg, 0.064mmol) and 0.1mL-0.5mL DMF was added, and the temperature was raised to 100°C-140°C to continue the reaction for 24h-48h. After the reaction was completed, the reaction solution was settled in 15 mL of ether, the precipitate was collected by centrifugation, and the remaining ether was removed by vacuum drying overnight. Dissolve the precipitate in H at a volume ratio of 10:1 2 In a mixed solvent of O and DMSO, and dialyzed in deionized water for 24 h with a dialysis bag with a molecular weight cut-off (MWCO) of 500, and then freeze-dried in a freeze dryer, the final product was a purple solid P-5O. The proton spectrum NMR characterization of gained polyporp...
Embodiment 3
[0027] Embodiment 3: the synthesis of water-soluble polyporphyrin P-11O
[0028] Accurately weigh tetracarboxyphenylporphyrin (25mg, 0.032mmol), EDCI (49mg, 0.256mmol) and DMAP (15.6mg, 0.128mmol) and dissolve in 1mL chloroform, heat to 60°C-80°C and stir for 10min. Then, a mixed solution of polyethylene glycol (32 mg, 0.064 mmol) with a molecular weight of 500 Da and 1 mL of chloroform was added, and the temperature was raised to 70° C. to continue the reaction for 24h-48h. After the reaction was completed, the reaction liquid was settled in 30 mL of n-hexane, the precipitate was collected by centrifugation, and the remaining n-hexane was removed by vacuum drying overnight. Dissolve the precipitate in 1 mL H 2 O, and dialyzed in deionized water for 24 h with a molecular weight cut-off (MWCO) bag of 1000, and then freeze-dried in a freeze dryer, the final product was purple solid P-11O.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com