Genetically modified stem cell for cartilage repair treatment and application thereof
A cartilage repair and gene modification technology, applied in the field of genetic engineering, can solve problems such as limited understanding of factors regulating its activity, effectiveness and safety limitations of clinical application of mesenchymal stem cells, etc., to achieve good synergistic effects, relieve reduction/avoidance Swelling, the effect of suppressing the activity of immune cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0058] According to a preferred embodiment of the present invention, the genetically modified stem cells further include an expression vector, and the nucleic acid encoding the anti-inflammatory factor and the nucleic acid encoding the cartilage repair factor are contained in the same or different expression vectors, preferably contained in the same expression vector. in the carrier.
[0059] In a further preferred embodiment, the nucleic acid encoding the anti-inflammatory factor and the nucleic acid encoding the cartilage repair factor are connected through a medium-strength promoter.
[0060] In a preferred embodiment of the present invention, the nucleic acid encoding the anti-inflammatory factor is linked to the nucleic acid encoding the cartilage repair factor, for example, through a medium-strength promoter.
[0061] More preferably, a medium-strength promoter such as EF1a is linked to an enhancer, the enhancer is linked to the 5' end of the nucleic acid encoding a cart...
Embodiment 1
[0121] Synthetic human XbaI-IL10-T2A-IL37-EF1α-5'LTR-FGF18-Sall
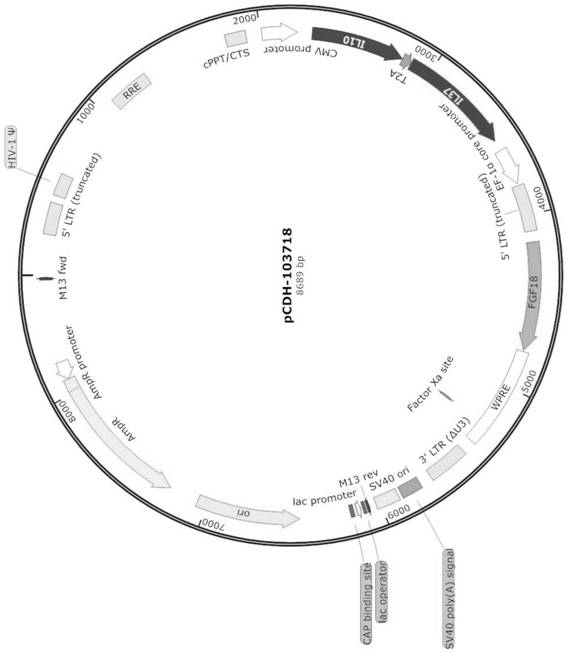
[0122] The IL10 coding nucleotide (the nucleotide sequence is shown in SEQIDNO:1) and the IL37 coding nucleotide (the nucleotide sequence is shown in SEQIDNO:2) are connected with T2A (the nucleotide sequence is shown in SEQIDNO:3) , FGF18 coding nucleotide (nucleotide sequence is shown in SEQIDNO:4) before connecting 5'LTR sequence (sequence shown in SEQIDNO:5) and EF1α promoter (sequence shown in SEQIDNO:7), IL37 and EF1α start The two ends were connected with restriction sites XbaI and SaII, and human XbaI-IL10-T2A-IL37-EF1α-5'LTR-FGF18-Sall was synthesized, named as Sequence103718, whose sequence is shown in SEQ ID NO:6.
Embodiment 2
[0123] Example 2 Construction of pCDH-103718 plasmid
[0124] The pCDH-CMV plasmid (Addgene) was digested with XbaI and SaII, and a fragment of about 6195 bp was recovered from the product gel as a vector.
[0125]Sequence103718 was digested with XbaI and SaII, and a fragment of about 2487bp was recovered from the product gel.
[0126] Take the pCDH-CMV plasmid digestion product vector and the Sequence103718 digestion product fragment, connect with T4 ligase overnight at 4°C, transform DH5α competent cells, take 100 μL of bacterial liquid and spread it on the LB plate containing ampicillin resistance, Cultivate overnight at 37°C, pick a single clone for colony PCR, send the positive clone to the sample for sequencing, save the clone with the correct sequencing result and extract the plasmid, named pCDH-103718, its map is as follows figure 1 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com