Preparation method and preparation device of deuterated low-carbon amine
A preparation device and deuterium technology are applied in the preparation field of chemical drug intermediates, which can solve the problems of difficulty in purchasing, large amount, high cost, etc., and achieve the effects of convenient quantification and subsequent operations, low moisture content, and high production efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] The preparation method of the deuterated lower carbon amine according to the embodiment of the present invention comprises:
[0070] Step S1, providing an inorganic acid salt solution of deuterated lower carbon amine;
[0071] Step S2, reacting the inorganic acid salt solution of the deuterated lower amine with a solid base to obtain the deuterated lower amine.
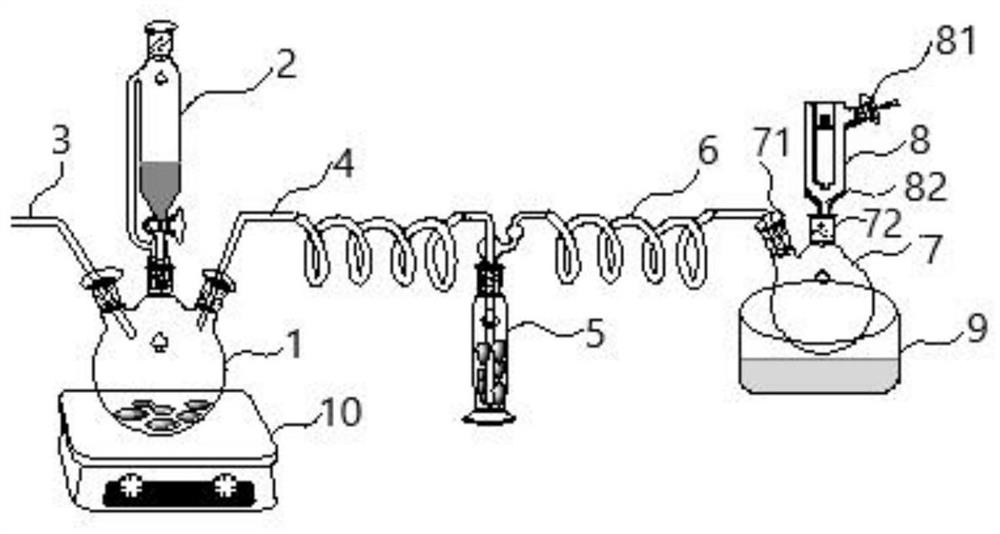
[0072] In combination with the above-mentioned preparation device, that is: through the constant pressure dropping funnel 2, dropwise add the inorganic acid salt solution of deuterated lower carbon amine to the three-necked bottle 1, so that the inorganic acid salt solution of deuterated lower carbon amine and the three-necked bottle 1 The solid base reacts to obtain free deuterated lower carbon amine.
[0073] This preparation method, for example, can be used to prepare the following deuterated lower amines: methyl-d 3 -amine, dimethyl-d 6 -amine, N-methyl-N-methyl-d 3 -amine,ethyl-d 5 -amine, diethyl-d ...
Embodiment 1
[0086] At room temperature, 2.0 g of methyl-d was dissolved using a constant pressure dropping funnel 3 - 5mL of deionized aqueous solution of amine hydrochloride, dripped on the flaky NaOH solid in the 25mL three-necked flask, a large amount of methyl-d will be produced immediately 3 - Amine gas.
[0087] in N 2 Carried by a carrier gas, methyl-d 3 - The amine gas passes through the gas dryer filled with granular KOH desiccant. After removing moisture, it enters the gas condensing device. It is cooled and liquefied in the micro-Dewar condenser with dry ice as the coolant. It is dropped into the dry ice-acetone Store in a 10 mL collector in the bath.
[0088] Weigh at low temperature and collect liquid methyl-d 3 -Amine 0.78g, yield: 80.4%.
Embodiment 2
[0090] At room temperature, 5.0 g of methyl-d was dissolved using a constant pressure dropping funnel 3 - 15mL deionized aqueous solution of amine sulfate, drop on the flaky KOH solid in the 50mL three-necked flask, a large amount of methyl-d will be produced immediately 3 - Amine gas.
[0091] in N 2 Carried by a carrier gas, methyl-d 3 - The amine gas passes through the gas dryer filled with granular KOH desiccant. After removing moisture, it enters the gas condensing device. It is cooled and liquefied in the micro-Dewar condenser with dry ice-ethanol as the coolant, and dropped into the liquid Store in a 10 mL collector in a nitrogen-acetone bath.
[0092] Weigh at low temperature and collect liquid methyl-d 3 -Amine 1.90g, yield: 92.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com