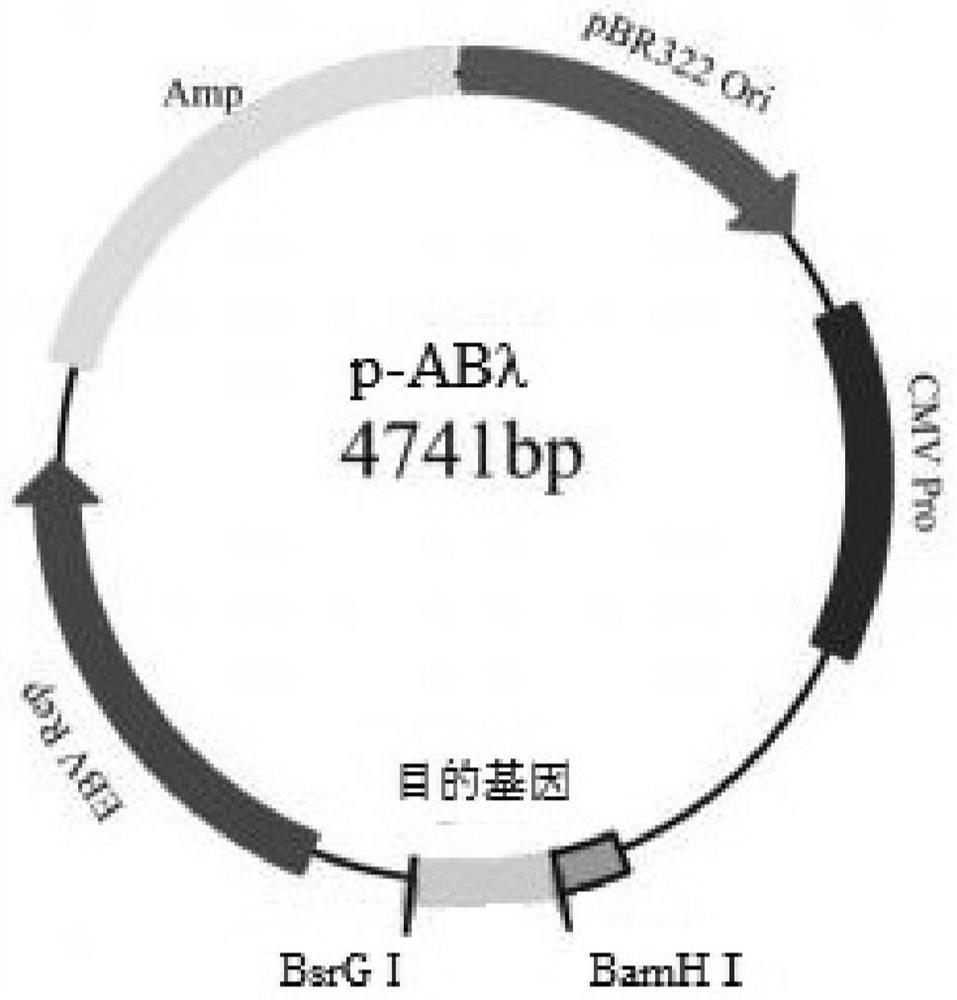
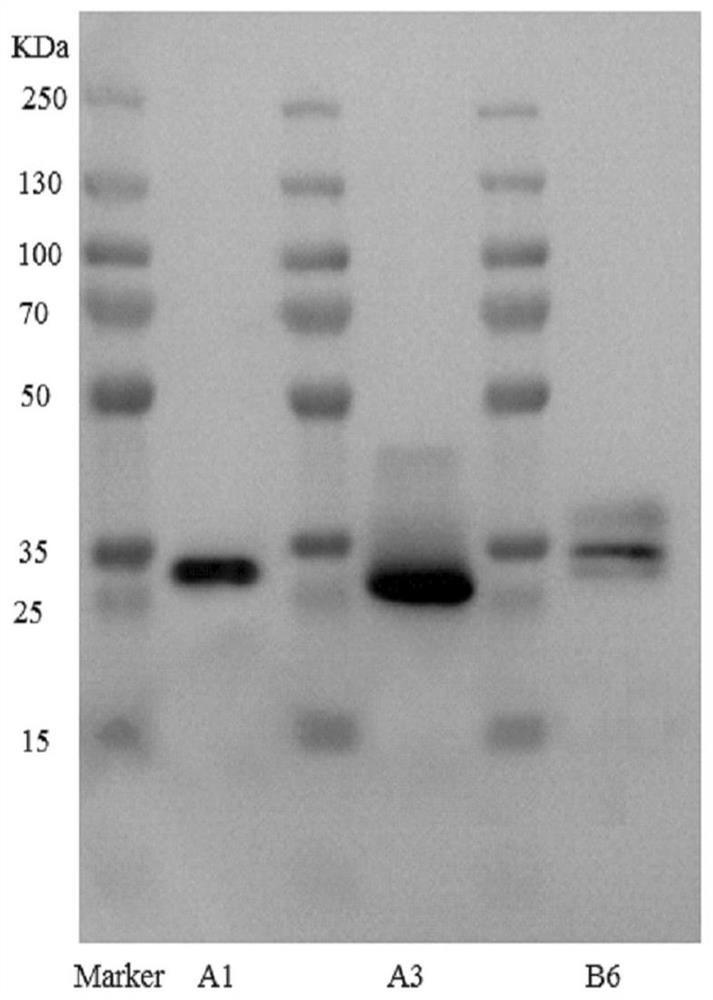
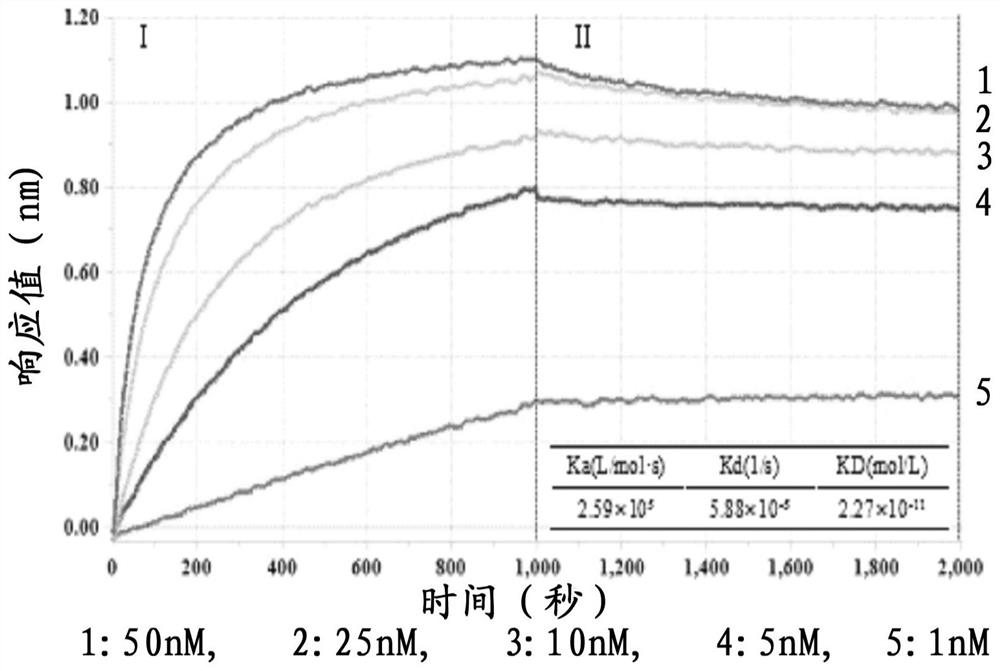
Targeted immunosuppressant TCABCD59 for preventing and treating infectious inflammation
A flexible and fusion protein technology, applied in the field of peptides, can solve the problems of lack of technical means, and achieve the effect of improving survival rate, reducing lung index and increasing survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1. Construction of human phage single-chain antibody C3d-ScFv
[0041] 1.1 For the construction of a large-capacity fully synthetic human phage single-chain antibody library, refer to Chinese patent 200910091261.8.
[0042] 1.2 Screening of human anti-human C3d single chain antibody
[0043] A total of three rounds of screening of human anti-human C3d single chain antibody
[0044] (1) Antigen coating: Human recombinant C3d protein-coated immunotubes were coated overnight at 4°C.
[0045] (2) Blocking: the immunotube was blocked with PBS containing 2% (w / v) BSA, and the phage antibody library was blocked with PBST (containing 0.1% Tween20) containing 2% (w / v) BSA at the same time, and blocked for 1 hour at 37°C.
[0046] (3) Binding: add the blocked phage antibody library into the immunotube, and let stand at 4°C overnight for binding;
[0047] (4) Washing: washing with PBST and PBS.
[0048] (5) Elution: Elution with 1 ml of 0.2 mol / l glycine-hydrochloric a...
Embodiment 2
[0074] Example 2. Construction and Identification of C3d-ScFv-CD59 Targeting Complement Inhibitor
[0075] Use PCR amplification technology to amplify single-chain antibody gene fragments and CD59 gene fragments; use upstream primers: B6F and downstream primers: B6-CD59-R to amplify single-chain antibody fragments (see Table 2 for primer sequences); use upstream primers CD59 -F, downstream primer: CD59-his-R amplifies the CD59 gene fragment. The PCR reaction system is the same as in Example 1.
[0076] Table 2. Construction of primer sequences targeting complement inhibitors
[0077]
[0078]The single-chain antibody fragment and the CD59 fragment were respectively connected to the upstream primer B6F by PCR technology, and the downstream primers were respectively CD59-his-R. PCR system (same as above). Expression and purification of the targeted complement inhibitor C3d-ScFv-CD59: the method is the same as in Example 1.
[0079] The lengths of the coding gene sequences...
Embodiment 3
[0084] Example 3. Serum Total Complement Hemolytic Activity (CH50) Determination
[0085] 3.1 Preparation of buffer
[0086] (1) Storage solution:
[0087] Na 2 HPO 4 12H 2 O 2.85g
[0088] K H 2 PO 4 0.27g
[0089] NaCl 17.00g
[0090] (Add distilled water to 100ml, store at 4°C)
[0091] (2) Application solution (buffer): add 95 ml of distilled water to 5 ml of stock solution, and add 0.1 ml of 10% magnesium sulfate. Prepared today. Use within 12 hours.
[0092] 3.2 Operation steps (improved Mayer method):
[0093] 1) Sensitized sheep erythrocytes: 2% sheep erythrocytes plus hemolysin (1:2000) after equal dilution, mix well, put in 37°C water bath for 30min.
[0094] 2) Diluted serum: 0.2ml of the serum to be tested was added with 3.8ml of buffer solution, and the dilution ratio was 1:20.
[0095] 3) Preparation of hemolysis standard tube: add 2ml of 2% sheep red blood cells to 8ml of distilled water, mix well, that is complete hemolysis. Take 2ml of total he...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Equilibrium dissociation constant | aaaaa | aaaaa |
| Equilibrium dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com