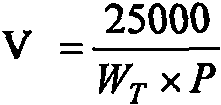Novel method for detecting titer of heparin sample by instrument
A heparin and potency technology, applied in the field of analysis of multi-component biochemical raw materials, can solve problems such as high personnel requirements, cumbersome methods, and improper operation control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
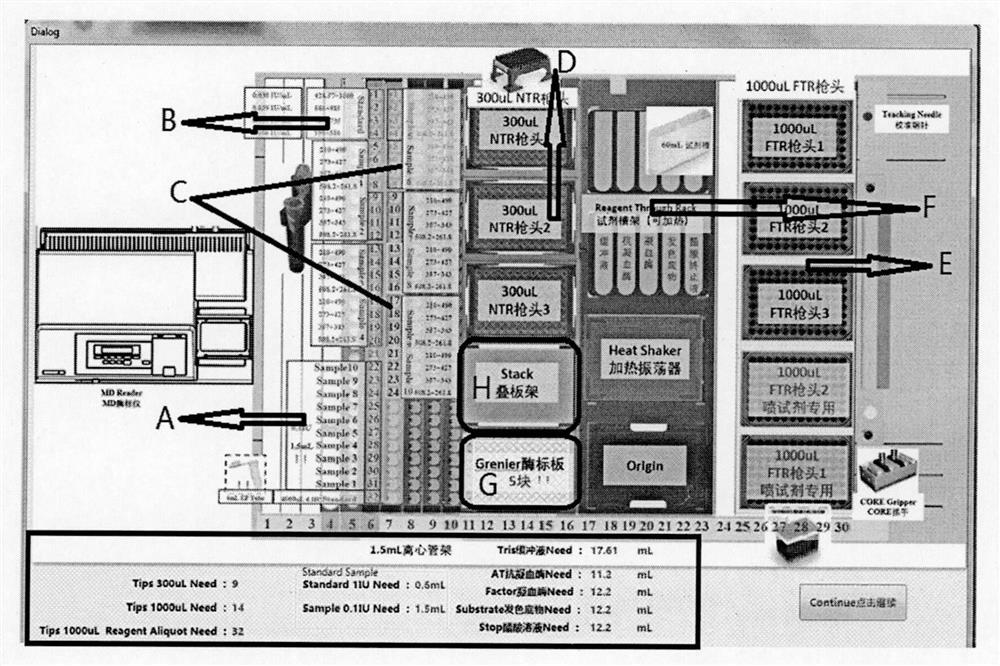
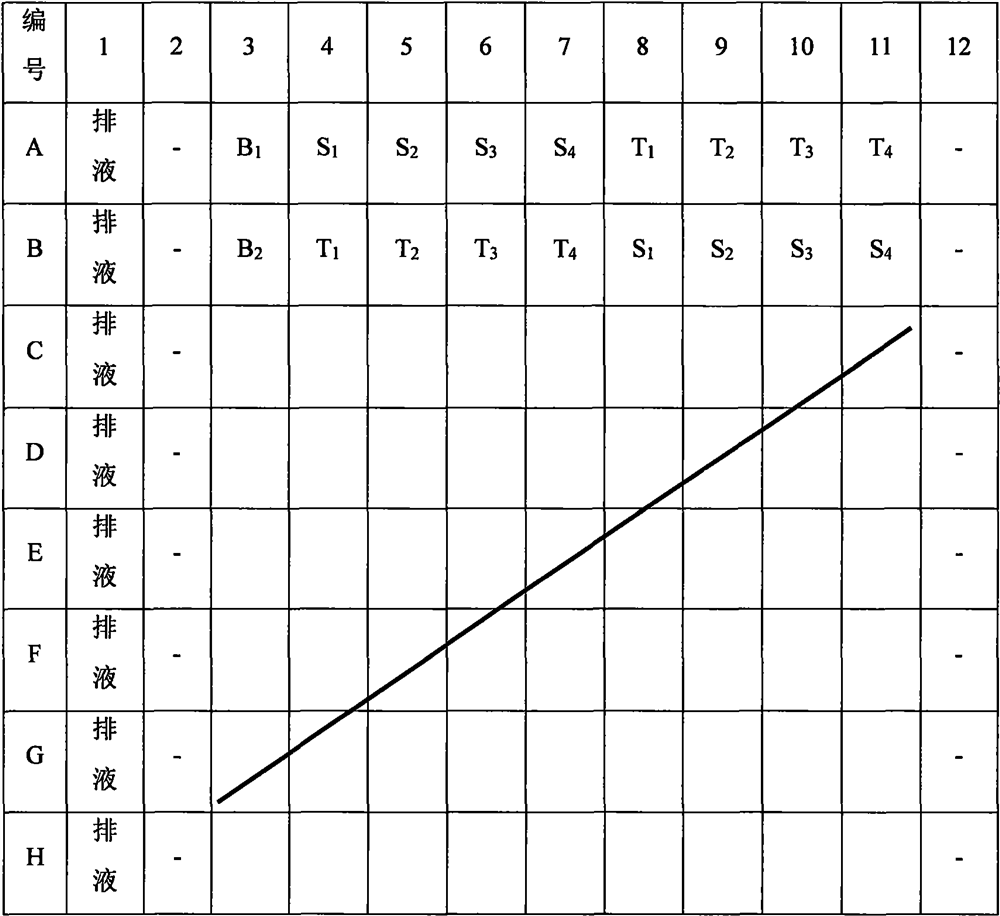
[0020] 1. Instruments and reagents
[0021] Fully automatic sample loading workstation, microplate reader, pipette, pH8.4 buffer, antithrombin, factor Xa, chromogenic substrate S-2765.
[0022] 2. Solution Preparation
[0023] 1) pH8.4 buffer solution: Weigh 6.06g of tris(hydroxymethyl)aminomethane, 2.8g of disodium edetate, 10.23g of sodium chloride, and 1.0g of polyethylene glycol 6000 and dissolve them in 800ml of water. / L hydrochloric acid to adjust the pH value to 8.4 and dilute to 1000ml.
[0024] 2) Antithrombin R1 solution: Add 10ml of pH 8.4 buffer to each bottle of antithrombin to obtain a solution containing 1.0IU / ml of antithrombin, and store it in a refrigerator at 2-8°C.
[0025] 3) Factor Xa solution: Add 12ml of pH8.4 buffer solution to each bottle to dilute to obtain 5.9nkat / ml factor Xa solution. Store in the refrigerator at 2-8°C.
[0026] 4) Chromogenic substrate S-2765 solution: add 11.66ml of water to each bottle of chromogenic substrate S-2765 reage...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com