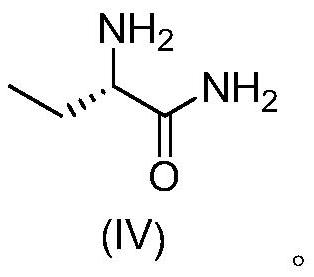
Synthesis method of (S)-2-aminobutanamide
A technology of aminobutyramide and a synthesis method, applied in the field of pharmaceutical synthesis, can solve the problems of low atom utilization rate, high environmental hazard, long process route, etc., and achieves high HPLC purity and optical purity, small amount of three wastes, and easy availability of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
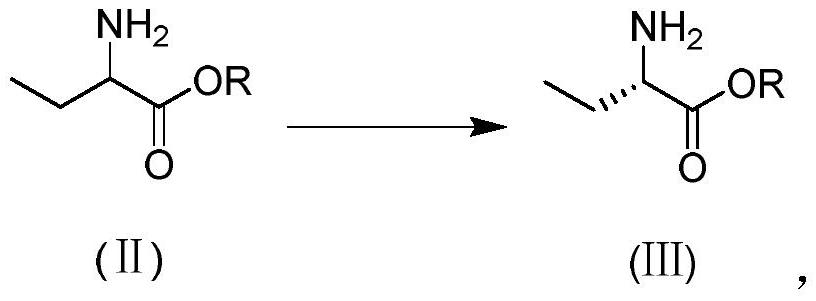
[0034] Enzymolysis reaction: add (R / S)-methyl 2-aminobutyrate (117g, 1mol) and H to the reaction flask 2 O (180g), heat up to 30°C under stirring, add immobilized bacterial agent (i.e. immobilized methylocystosis, preservation number is CCTCC NO: M2016494) (10g), control the temperature in the range of 25-35°C Inside, add dropwise 20% Na 2 CO 3 solution, keep the pH of the reaction system at 6.5 to 8.0, react until the isomer (R)-2-aminobutyric acid methyl ester is less than 1%, stop the reaction, filter, reclaim the immobilized bacterial agent, add the extractant toluene ( 200g), stirred for 30 minutes, stood for 30 minutes, separated layers, collected the organic phase, repeated the extraction operation 3 times, combined the organic phase, added anhydrous magnesium sulfate (20g) to the organic phase, stirred and dried for 30 minutes, filtered, collected Filtrate, carry out vacuum distillation to filtrate, control vacuum distillation temperature 50~60 ℃, vacuum distillation...
Embodiment 2
[0037] Enzymolysis reaction: add (R / S)-methyl 2-aminobutyrate (117g, 1mol) and H to the reaction flask 2O (120g), heat up to 30°C under stirring, add immobilized bacterial agent (i.e. immobilized methylocystosis, preservation number is CCTCC NO: M2016494) (6g), control the temperature in the range of 25-35°C Inside, add 20% K dropwise 2 CO 3 solution, keep the pH of the reaction system at 6.5 to 7.5, react until the isomer (R)-2-aminobutyric acid methyl ester is less than 1%, stop the reaction, filter, reclaim the immobilized bacterial agent, add the extractant toluene ( 150g), stirred for 30 minutes, stood still for 30 minutes, separated layers, collected the organic phase, repeated the extraction operation 3 times, combined the organic phase, added anhydrous sodium sulfate (20g) to the organic phase, stirred and dried for 30 minutes, filtered, collected Filtrate, the filtrate was subjected to vacuum distillation, controlled vacuum distillation temperature 50 ~ 60 ° C, vacu...
Embodiment 3
[0040] Enzymolysis reaction: add (R / S)-methyl 2-aminobutyrate (117g, 1mol) and H to the reaction flask 2 O (150g), heat up to 30°C under stirring, add immobilized bacterial agent (i.e. immobilized methylocystosis, preservation number is CCTCC NO: M2016494) (12g), control the temperature in the range of 25-35°C Inside, add dropwise 10% Na 2 CO 3 Solution, keeping the pH of the reaction system at 7.0 to 8.0, reacting to stop the reaction when the isomer (R)-2-aminobutyric acid methyl ester is less than 1%, filtering, reclaiming the immobilized bacterial agent, adding the extractant toluene ( 200g), stirring for 30 minutes, standing for 30 minutes, layering, collecting the organic phase, repeating the extraction operation 3 times, merging the organic phase, adding anhydrous sodium sulfate (20g) to the organic phase, stirring and drying for 30 minutes, filtering, collecting Filtrate, the filtrate was subjected to vacuum distillation, controlled vacuum distillation temperature 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com