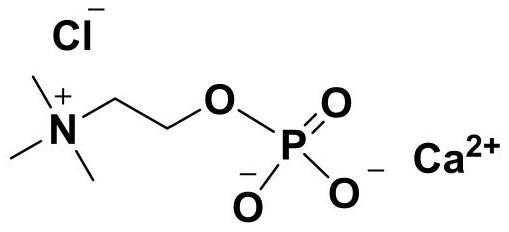
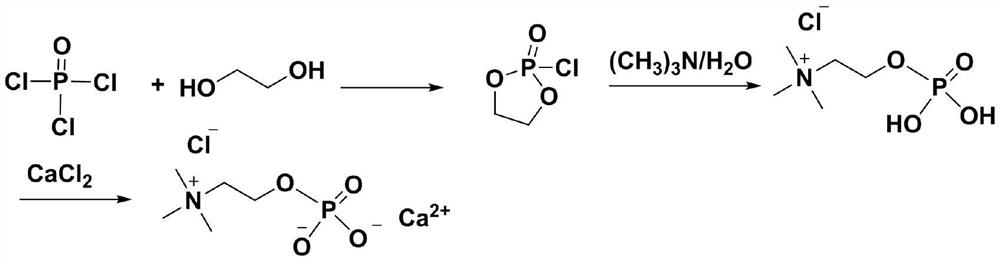
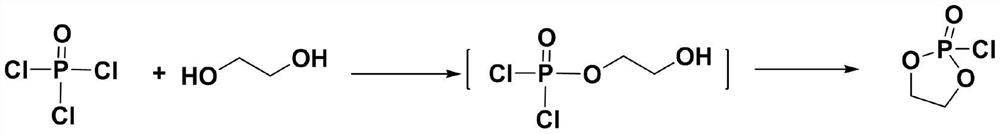
Preparation method of calcium phosphorylcholine chloride
A technology for phosphoryl choline chloride and calcium chloride, which is applied in the field of preparation of calcium phosphoryl choline chloride, and can solve the problems of environmental pollution, difficulty in removing phosphorylation reagents or condensing agents, and easy moisture absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In the reaction flask, add dichloromethane (5mL), triethylamine (0.28mL, 2mmol) and phosphorus oxychloride (0.093mL, 1mmol), cool to -5 ~ 0 ℃ with ice brine, dropwise add ethylene glycol ( 0.056mL, 1mmol), the dropwise addition was completed, stirred and continued to react for 30min, then slowly raised to room temperature, continued to react for 2h, filtered to remove the formed triethylamine hydrochloride, concentrated the filtrate under reduced pressure below 30°C, added toluene to azeotropically remove HCl remained, and 0.135 g of a viscous oil was obtained, namely the intermediate 2-chloro-1,3,2-dioxaphospholane-2-oxide, with a yield of 95%. 1 H NMR (300MHz, CDCl 3 )δ4.41-4.23(m,4H,O-CH 2 -CH 2 -O). 13 CNMR (100MHz, D 2 O) δ71.1.
Embodiment 2
[0030] In the reaction flask, add dichloromethane (50mL), triethylamine (2.78mL, 20mmol) and phosphorus oxychloride (0.93mL, 10mmol), cool to -5 ~ 0 ℃ with ice brine, dropwise add ethylene glycol ( 0.56mL, 10mmol), the dropwise addition was completed, stirred and continued to react for 30min, then slowly raised to room temperature, continued to react for 2h, filtered to remove the formed triethylamine hydrochloride, concentrated the filtrate under reduced pressure below 30°C, added toluene to azeotropically remove HCl remained, and 1.39 g of a viscous oil was obtained, namely the intermediate 2-chloro-1,3,2-dioxaphospholane-2-oxide, with a yield of 98%.
Embodiment 3
[0032] In the reaction flask, add dichloromethane (5mL), triethylamine (0.28mL, 2mmol) and phosphorus oxychloride (0.093mL, 1mmol), cool to -5 ~ 0 ℃ with ice brine, dropwise add ethylene glycol ( 0.028mL, 0.5mmol), the dropwise addition was completed, stirred and continued to react for 30min, then slowly raised to room temperature, continued to react for 2h, filtered to remove the formed triethylamine hydrochloride, concentrated the filtrate under reduced pressure below 30°C, added toluene to azeotrope Residual HCl was removed to obtain 0.067g viscous oil, which was the intermediate 2-chloro-1,3,2-dioxaphospholane-2-oxide compound, yield 95% (taking ethylene glycol as standard calculate).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com