Poly(2-methacryloyloxyethyl phosphorylcholine) entrapped nimotuzumab nanocapsule, as well as preparation method and application thereof
A technology of methacryloyloxyethylphosphorylcholine and nimotuzumab, which is applied in the direction of microcapsules, nanocapsules, chemical instruments and methods, and can solve the problems of short blood drug half-life, immunogenicity, and large molecular weight. and other problems, to achieve the effect of avoiding toxic and side effects, inhibiting proliferation, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
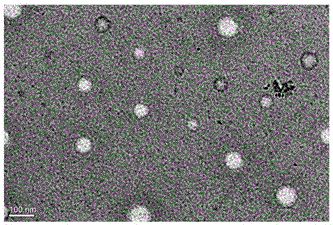
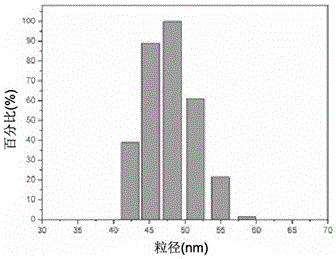
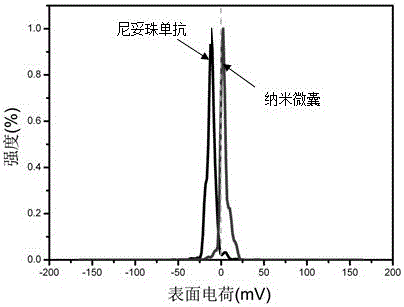
[0026] Example 1 Preparation and characterization of nimotuzumab nanocapsules encapsulated by poly-2-methacryloyloxyethyl phosphocholine
[0027] Take a solution containing 1 mg of Nimotuzumab (Baitai Biopharmaceutical Co., Ltd.), add the positive monomer N-(3-aminopropyl) methacrylate, Nimotuzumab and N-(3) The molar ratio of -aminopropyl) methacrylate is 1:300, and then add 2-methacryloxyethyl phosphocholine, nimotuzumab and 2-methacryloxyethyl phosphocholine The molar ratio of nimotuzumab to the cross-linking agent is 1:500; then the matrix metalloproteinase-2 degradable peptide cross-linking agent is added at the ratio of nimotuzumab to the cross-linking agent at a molar ratio of 1:500. Hydrogen bonding to enrich the reaction monomer and enzyme-degradable peptide cross-linking agent around the monoclonal antibody; then add ammonium persulfate and tetramethylethylenediamine, nimotuzumab and ammonium persulfate, tetramethylethyl The molar ratio of the diamine was 1:500:1000, a...
Embodiment 2
[0029] Example 2: Evaluation of the therapeutic effect of nimotuzumab nanocapsules coated with poly-2-methacryloxyethyl phosphocholine on U87 glioma in situ model in nude mice
[0030] The day before virus infection, U87 cells (ATCC, USA, HTB-14) were plated on a 24-well plate with a density of 1×10 5 -1×10 6 , At 5% CO 2 , Constant temperature culture at 37℃, the medium is DMEM medium (GBICO, USA, 11965-092), and the serum is imported calf serum (HyClone, SH30071.03). After 24h, add 10-500μl of virus solution encoding luciferase (Shanghai Jima Pharmaceutical Technology Co., Ltd.), and add 10-50μl of polybrene to increase the infection efficiency; after 12h, change to DMEM medium; after 48h Add the screening drug 10-100μl Puromycin, and after 2 weeks of pressure screening, positive clones are obtained, and the culture is expanded to prepare for transplantation into nude mice to establish an orthotopic model.
[0031] After the nude mouse is anesthetized and stabilized, make a surgi...
Embodiment 3
[0033] Example 3: Evaluation of the therapeutic effect of nimotuzumab nanocapsules coated with poly-2-methacryloxyethyl phosphocholine on the subcutaneous model of MGC803 gastric cancer in nude mice
[0034] Use a 100μl micro-injector to transfer MGC803 gastric cancer cells stably expressing luciferase to 5×10 per injection point. 5 The cells were inoculated subcutaneously in 4-week-old nude mice to establish the tumor source. When the subcutaneous tumor reached 3cm in length, the tumor mass was removed, evenly chopped, and implanted subcutaneously in nude mice of each experimental group (n=6), and feeding continued. When the long diameter reached about 5mm, each experimental group started treatment. The treatment method is tail vein injection, the treatment dose is 5 mg per kilogram of body weight, and the number of treatments is once. At the same time, the treatment effect was observed. The luciferase activity value of the tumor cells was collected by the in vivo imager every t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com