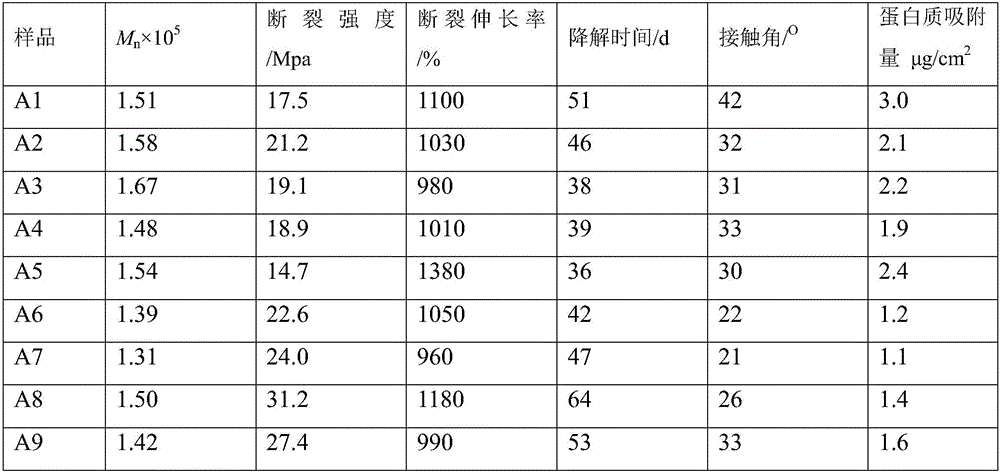
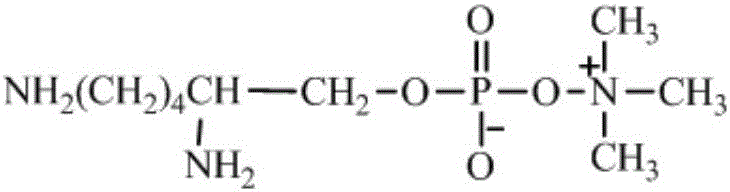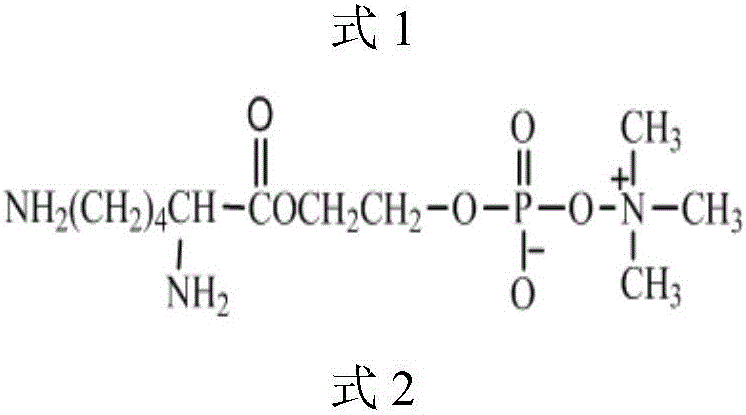High-biocompatibility phosphorylcholine-modified polyurethane material and prepration method thereof
A technology of biocompatibility and polyurethane materials, which is applied in the field of highly biocompatible phosphorylcholine modified polyurethane materials and its preparation, can solve the problems of limited application, complexity, and difficulty in enriching the surface, so as to improve biological Effect of compatibility, good mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of double-terminated hydroxyl prepolymer:
[0039] Put 15g (0.05mol) of PEG300 in a vacuum reaction flask, stir with a magnet, remove water in a vacuum (30Pa) at a reaction temperature of 100°C for 4 hours, cool to room temperature, and balance nitrogen into the vacuum reaction flask. Then add 60g of L-lactide and 0.3g of dibutyltin diacetate in the vacuum reaction bottle. Then vacuumize the vacuum reaction bottle, and then pass dry nitrogen to balance, repeat three times. Finally, evacuate to 30Pa, seal the vacuum reaction bottle, heat the oil bath to 130°C, and react for 24 hours to obtain the double-terminated hydroxyl prepolymer poly L-lactide-polyethylene glycol-poly L-lactide (PLLA- PEG-PLLA, M n = 1500, PEGwt% = 20%).
[0040] (2) Preparation of double-terminated isocyanate-based prepolymers
[0041] Place 15g (0.01mol) of PLLA-PEG-PLLA prepared in (1) and 2.52g (0.015mol) of 1,6-hexamethylene diisocyanate in a three-necked flask, protect it w...
Embodiment 2
[0047] (1) Preparation of double-terminated hydroxyl prepolymer:
[0048]Put 10.0g (0.05mol) of PEG200 in a vacuum reaction bottle, stir with a magnet, and remove water under vacuum (30Pa) at a reaction temperature of 100°C for 4 hours, then cool to room temperature, and pass nitrogen gas into the vacuum reaction bottle for balance. Then add 40g ε-caprolactone and 0.2g stannous octoate into the vacuum reaction bottle. Then vacuumize the vacuum reaction bottle, and then pass dry nitrogen to balance, repeat three times. Finally, vacuumize to 30Pa, seal the vacuum reaction bottle, heat the oil bath to 140°C, and react for 24 hours to obtain the double-terminated hydroxyl prepolymer polyε-caprolactone-polyethylene glycol-polyε-caprolactone (PCL- PEG-PCL, M n = 1000, PEGwt% = 20%).
[0049] (2) Preparation of double-terminated isocyanate-based prepolymers
[0050] Put 10g (0.01mol) of PCL-PEG-PCL prepared in (1) and 3.39g (0.015mol) of L-lysine diisocyanate in a three-necked fl...
Embodiment 3
[0056] (1) Preparation of double-terminated hydroxyl prepolymer:
[0057] Put 10.0g (0.05mol) of PEG200 in a vacuum reaction bottle, stir with a magnet, and remove water under vacuum (30Pa) at a reaction temperature of 100°C for 4 hours, then cool to room temperature, and pass nitrogen gas into the vacuum reaction bottle for balance. Then add 40g ε-caprolactone and 0.2g stannous octoate into the vacuum reaction bottle. Then vacuumize the vacuum reaction bottle, and then pass dry nitrogen to balance, repeat three times. Finally, evacuate to 30Pa, seal the vacuum reaction bottle, heat the oil bath to 130°C, and react for 36 hours to obtain the double-terminated hydroxyl prepolymer poly(L-lactide-glycolide)-polyethylene glycol-poly(L - lactide-glycolide) (PLGA-PEG-PLGA, M n = 1000, PEGwt% = 20%).
[0058] (2) Preparation of double-terminated isocyanate-based prepolymers
[0059] Put 10g (0.01mol) of PLGA-PEG-PLGA prepared in (1) and 3.39g (0.015mol) of L-lysine diisocyanate i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com