Exbead for removing inorganic acid and manufacturing method therefor
A technology of inorganic acids and beads, applied in separation methods, chemical instruments and methods, transportation and packaging, etc., can solve the problems of limited neutralization efficiency, problems with removal efficiency, inaccessibility, etc., and achieve excellent morphological stability, The effect of improving workability and increasing the degree of crushing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
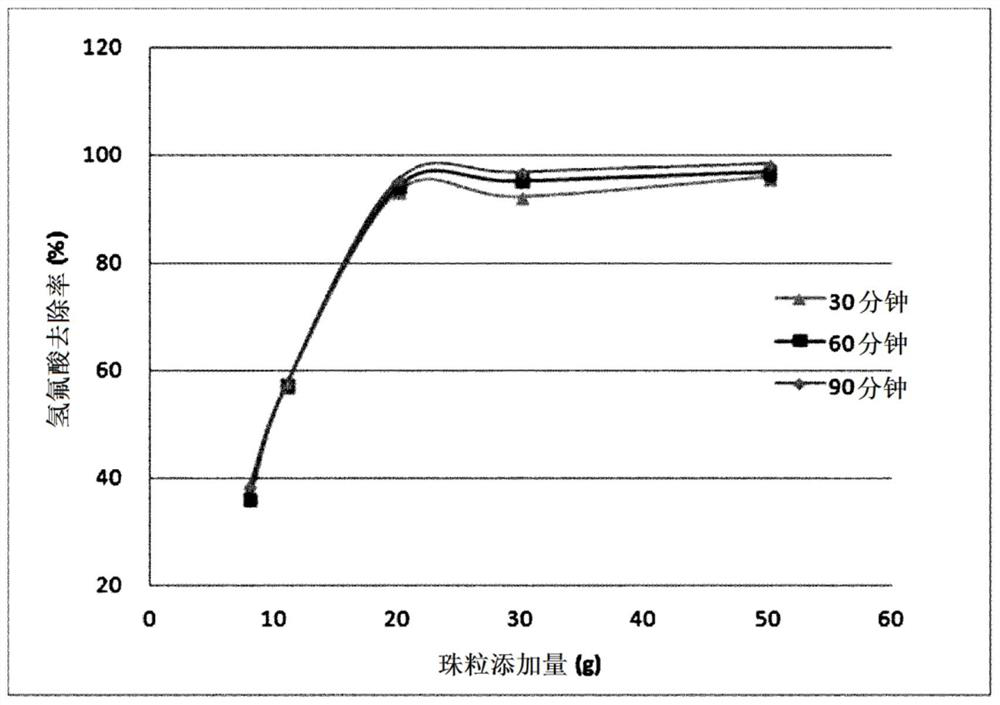
[0071] Kaolin was put into a circular drum and rotated at 80 rpm for 9 hours to produce clay seed particles (moisture content: 10% by weight) with an average particle diameter of 2.0 mm. The produced seed particles were placed in a circular drum, and a powder obtained by mixing 33 parts by weight of slaked lime and 5 parts by weight of clay was added to 100 parts by weight of sodium bicarbonate to obtain a mixed raw material powder. The composition ratio of the above-mentioned seeds (excluding water content) and the above-mentioned mixed raw material powder was mixed at a weight ratio of 23:77. Next, while spraying 20 parts by weight of water with respect to 100 parts by weight of the raw material powder, the circular drum was rotated at 80 rpm to form a nucleus layer containing sodium bicarbonate, slaked lime and a clay binder on the seed surface. Granule-type beads having an average particle size of the formed beads of 3.4 mm were obtained, which were dried in an oven at 60°...
Embodiment 2
[0075] In the above-mentioned Example 1, after manufacturing the finally manufactured beads, with respect to 100 parts by weight of the manufactured beads, 100 parts by weight of calcium hydroxide, 20 parts by weight of kaolin and 10 parts by weight of flour were added to the shell layer forming composition, While spraying 15 parts by weight of water to 100 parts by weight of the added composition, the circular drum was rotated at 100 rpm to form a shell layer on the surface of the core particles, thereby producing beads for hydrofluoric acid removal. Then, the manufactured beads were put into a ceramic container, placed in an oven, and subjected to heat treatment at 60° C. for 30 hours, and beads were finally obtained. The average particle size of the final beads was 3.7 mm. As a result of the hydrofluoric acid removal rate test carried out by the same method as in Example 1 above, it can be confirmed that only 20 g is added to 10 ml of 52% hydrofluoric acid aqueous solution,...
Embodiment 3
[0077] In Example 2, when a mixture of bicarbonate and slaked lime was used to form the core layer, 3 parts by weight of tetraethoxysilane and 3-aminopropyltrimethoxy The same experiment as in Example 2 was carried out except that the base silane was used. As a result of the hydrofluoric acid removal rate test carried out by the same method as in Example 1 above, it can be confirmed that only 20 g is added to 10 ml of 52% hydrofluoric acid aqueous solution, and a reaction time of 30 minutes shows a hydrofluoric acid removal rate of about 97%. . In addition, when the manufactured inorganic acid remover was subjected to 100 drop tests at a height of 1 m, compared with Example 2, the bead breakage rate was 8% by weight, and compared with 11% by weight in Example 2, it can reach a significant Elevated effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com