Zinc gluconate oral solution and preparation method thereof
A zinc gluconate and oral solution technology, which is applied in the field of medicine, can solve problems such as bad taste, human injury, and influence on consumer use, and achieve the effects of improving product taste, increasing osmotic pressure, and improving product safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
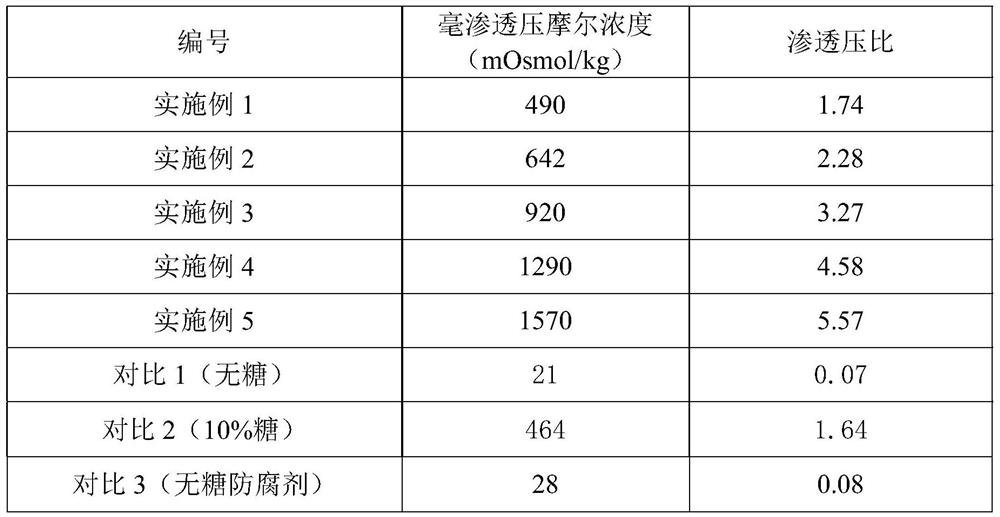
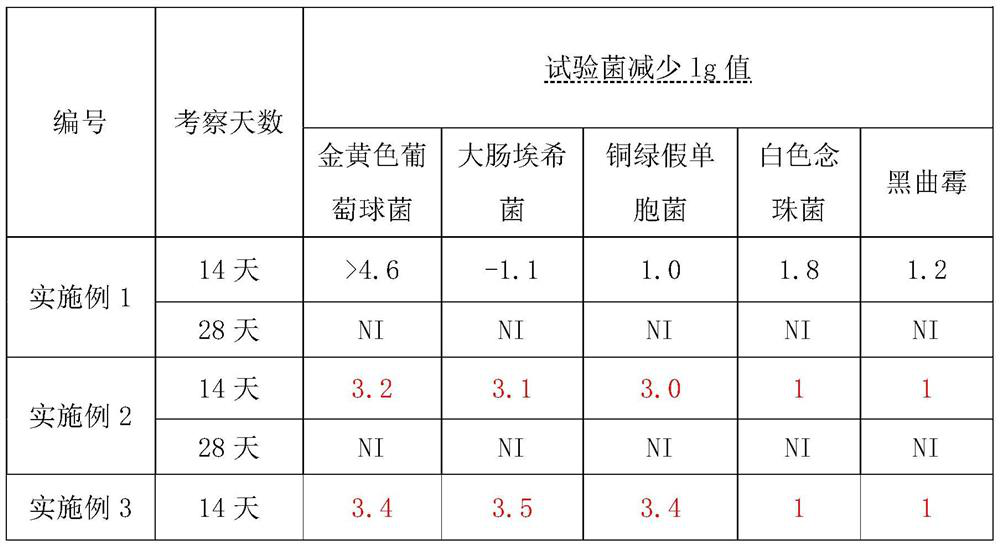
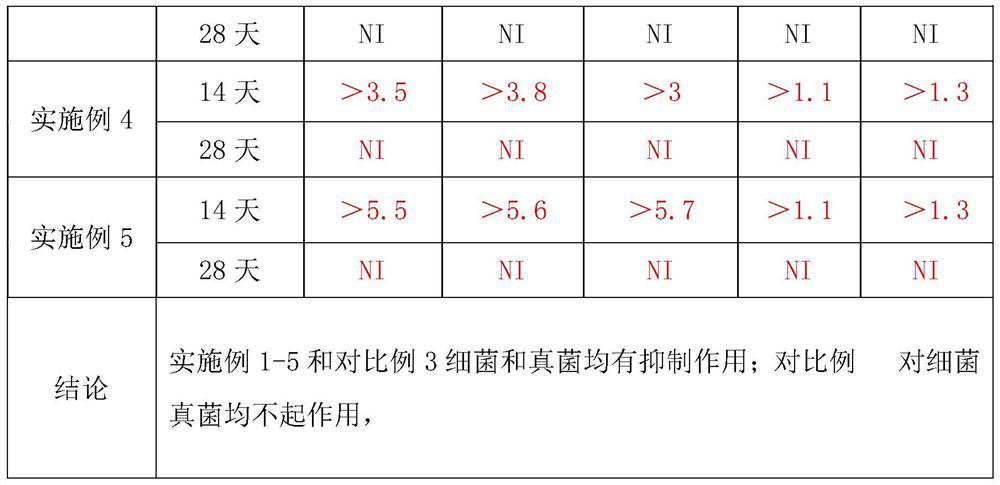
Image
Examples
Embodiment 1
[0018] (1) Take 3000ml of purified water, heat to boil, add 30g of zinc gluconate under stirring, dissolve and heat to boiling;
[0019] (2) Weigh 1500g of sucrose, slowly add in (1) boiling solution, stir evenly, boil for 20min, stop heating, cool down, set aside;
[0020] (3) Weigh 3 g of citric acid, add purified water to prepare a 30% citric acid solution, and sterilize it at 105° C. for 30 minutes in an airtight manner before use.
[0021] (4) Add (3) to the solution in (2), add an appropriate amount of purified water, adjust the pH value to 3.0-5.0, add purified water to 10000ml, stir well, and fine filter through a 5 μm titanium rod and a 1 μm folded membrane;
[0022] (5) Filling after filtration, airtight sterilization at 100-110°C for 50 minutes.
Embodiment 2
[0024] (1) Take 3500ml of purified water, heat to boil, add 35g of zinc gluconate under stirring, dissolve and heat to boiling;
[0025] (2) Weigh 2000g of sucrose, slowly add in (1) boiling solution, stir evenly, boil for 60min, stop heating, cool down, and set aside;
[0026] (3) Weigh 2.5 g of citric acid, add purified water to prepare a 30% citric acid solution, and sterilize it at 105° C. for 30 minutes in an airtight manner before use.
[0027] (4) Add (3) to the solution in (2), add an appropriate amount of purified water, adjust the pH value to 3.0-5.0, add purified water to 10000ml, stir well, and fine filter through a 5 μm titanium rod and a 1 μm folded membrane;
[0028] (5) Filling after filtration, airtight sterilization at 100-110°C for 60 minutes.
Embodiment 3
[0030] (1) Take 4000ml of purified water, heat to boil, add 40g of zinc gluconate under stirring, dissolve and heat to boiling;
[0031] (2) Weigh 3000g of sucrose, slowly add it into (1) boiling solution, stir evenly, boil for 40min, stop heating, cool down, and set aside;
[0032] (3) Weigh 2.5 g of citric acid, add purified water to prepare a 30% citric acid solution, and sterilize it at 105° C. for 30 minutes in an airtight manner before use.
[0033] (4) Add (3) to the solution in (2), add an appropriate amount of purified water, adjust the pH value to 3.0-5.0, add purified water to 10000ml, stir well, and fine filter through a 5 μm titanium rod and a 1 μm folded membrane;
[0034] (5) Filling after filtration, airtight sterilization at 100-110°C for 50 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com