6, 7-disubstituted 2-(ethylthio)-pteridine-4-amine derivative and preparation method and application thereof
A technology of amine derivatives and ethylthio, which is applied in the field of 2--pteridine-4-amine derivatives and its preparation, can solve the problems of unsuitability for large-scale industrial production, high synthesis difficulty, and many steps, and achieve broad Biomedical application prospects, good anti-tumor activity, and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] The preparation of embodiment 1 compound 1a
[0066]
[0067] The preparation method of the compound 1a comprises the following steps:
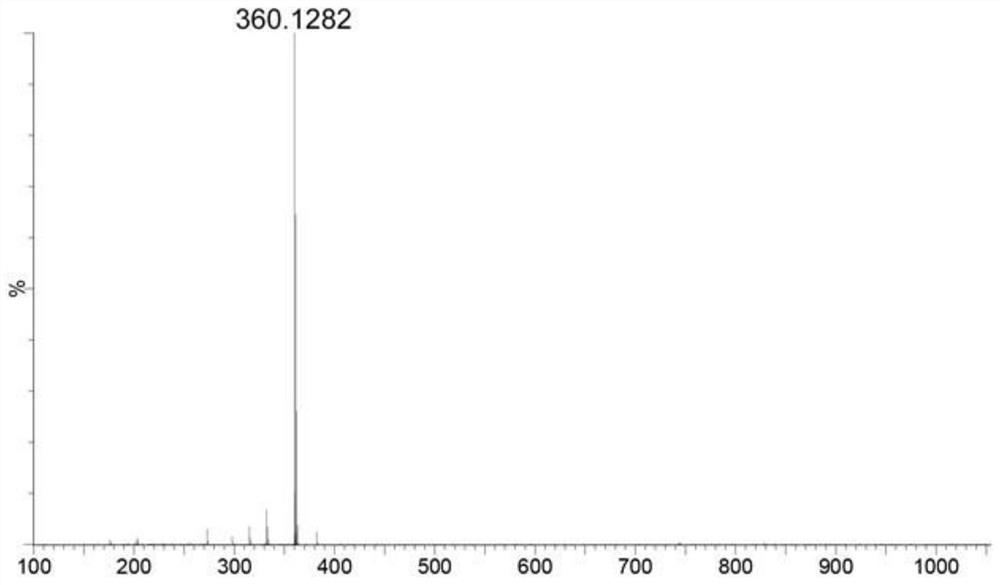
[0068] Add 1 mol of 2-(ethylthio)pyrimidine-4,5,6-triamine and 1 mol of benzil to 12 mL of DMF, stir and heat to 110°C for 5 hours, cool to room temperature, and dissolve the reaction solution Add it into 80 mL of saturated NaCl aqueous solution, stir thoroughly for half a minute, filter, wash the filter cake twice with ultrapure water (10 mL×2), and freeze-dry to obtain the crude product, which is separated and purified by column chromatography, and the mobile phase is CH 2 Cl 2 :CH 3 OH=30:1 (V / V). The target product 1a was obtained with a yield of 55%.
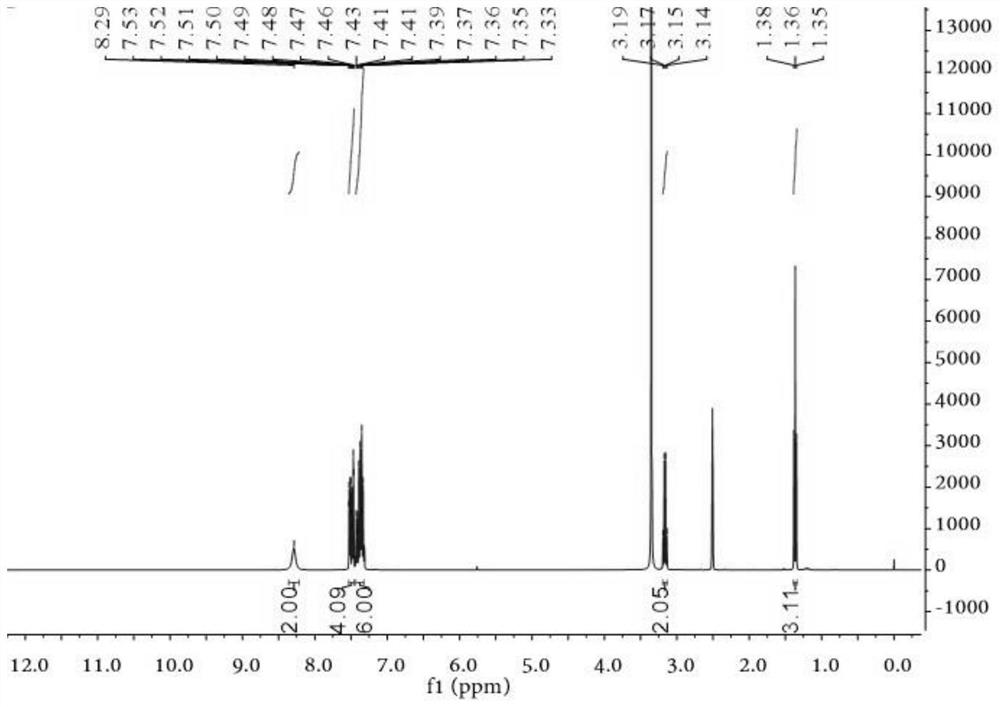
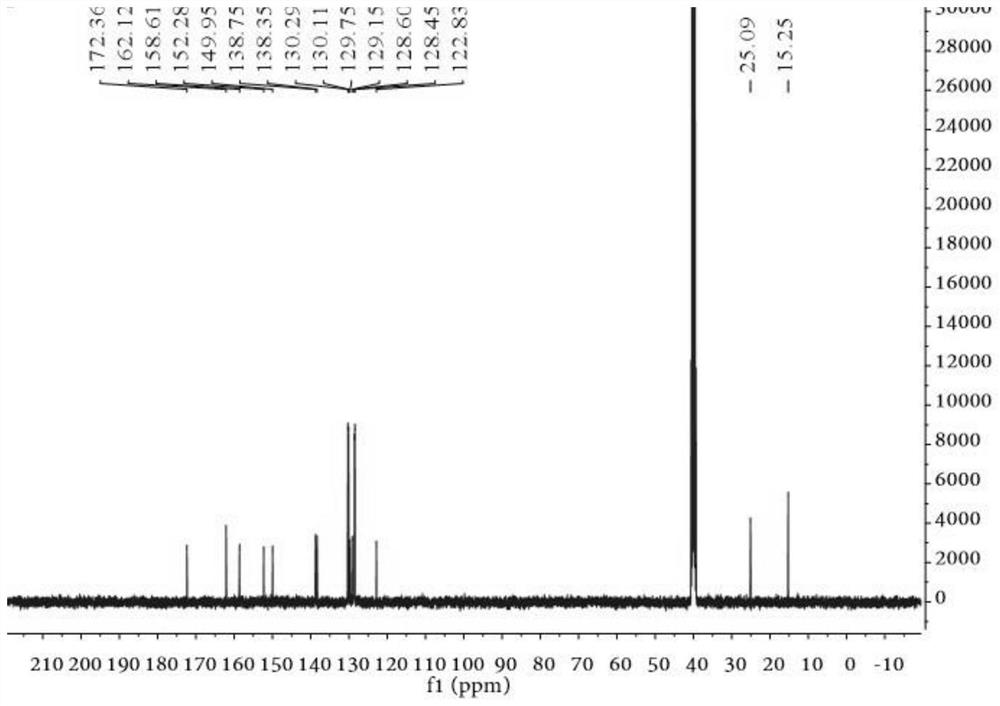
[0069] 1 H NMR (400MHz, DMSO-d 6 )δ:8.29(s,2H,NH 2 ), 7.53-7.46 (m, 4H, ArH); 7.43-7.33 (m, 6H, ArH), 3.15 (q, J = 7.31Hz, 2H, CH 2 ), 1.36(t, J=7.28Hz, 3H, CH 3 ); 13 C NMR (100MHz, DMSO-d 6 )δ: 172.36, 162.12, 158.61, 152.28, 149.95, 138.75, 138.35, 130.29, 130.11, 1...
Embodiment 12
[0083] The preparation of embodiment 12 compound 1b
[0084]
[0085] The preparation method of the compound 1b comprises the following steps:
[0086] Add 1 mol of 2-(ethylthio)pyrimidine-4,5,6-triamine and 1 mol of 4,4'-difluorobenzil into 12 mL of DMF, stir and heat to 110°C for 5 hours, Cool to room temperature, add the reaction solution to 80 mL of saturated NaCl aqueous solution, stir thoroughly for half a minute, filter, wash the filter cake twice with ultrapure water (10 mL×2), and freeze-dry to obtain the crude product, which is separated and purified by column chromatography , the mobile phase is CH 2 Cl 2 :CH 3 OH=30:1 (V / V), the target product 1b was obtained with a yield of 51%.
[0087] 1 H NMR (400MHz, DMSO-d 6 )δ:8.31(s,2H,NH 2 ),7.55(m,4H,ArH),7.22(m,4H,ArH),3.15(q,J=7.30Hz,2H,CH 2 ), 1.36(t, J=7.29Hz, 3H, CH 3 ); 13 C NMR (100MHz, DMSO-d 6 )δ:172.49,164.35,164.06,162.07,161.90,161.61,157.54,152.26,148.89,135.11,134.71,132.63,132.57,132.49,122.84...
Embodiment 13
[0089] Preparation of Example 13 Compound 1c
[0090]
[0091] The preparation method of the compound 1c comprises the following steps:
[0092] Add 1 mol of 2-(ethylthio)pyrimidine-4,5,6-triamine and 1 mol of furil to 12 mL of DMF, stir and heat to 110°C for 5 hours, cool to room temperature, and dissolve the reaction solution Add it into 80mL of saturated NaCl aqueous solution, stir thoroughly for half a minute, filter, wash the filter cake twice with distilled water (10mL×2), and freeze-dry to obtain the crude product, which is separated and purified by column chromatography, and the mobile phase is CH 2 Cl 2 :CH 3 OH=30:1 (V / V), the target product 1c was obtained with a yield of 56%.
[0093] 1 H NMR (400MHz, DMSO-d 6 )δ:8.30(s,1H,NH),8.16(s,1H,NH),7.88(m,1H,ArH),7.80(m,1H,ArH),6.98(dd,J=0.83,3.42Hz, 1H, ArH), 6.70(dd, J=0.75, 3.72Hz, 1H, ArH), 6.68~6.66(m, 2H, ArH), 3.11(q, J=7.28Hz, 2H, CH 2 ), 1.32(t, J=7.32Hz, 3H, CH 3 ); 13 C NMR (100MHz, DMSO-d 6 )δ: 173...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com