Formononetin derivative as well as preparation method and application thereof
A kind of technology of formononetin and derivatives, applied in the field of formononetin derivatives and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
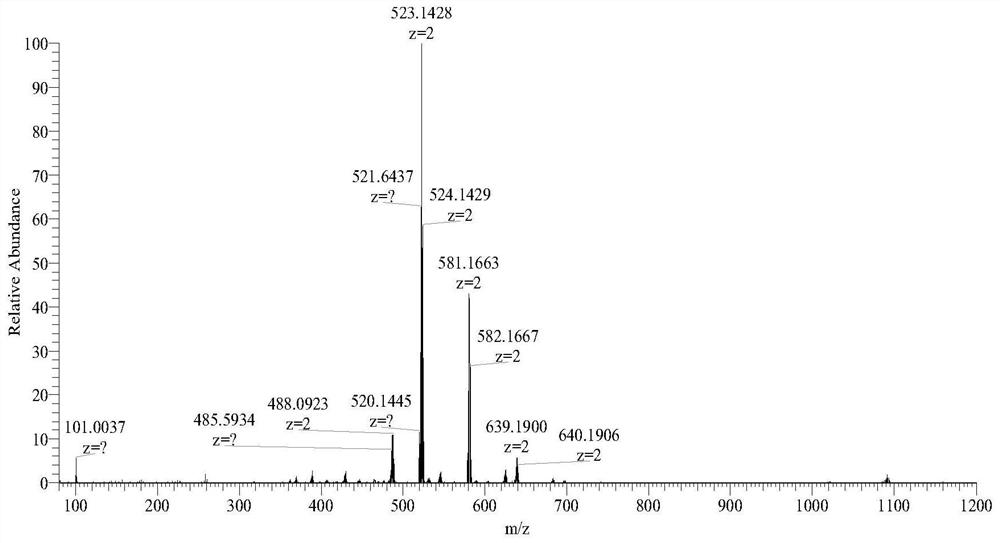
Image
Examples
preparation example Construction
[0050] The present invention also provides a preparation method for formononetin derivatives described in the above technical scheme, comprising the following steps:
[0051] mixing formononetin, ruthenium complex, potassium carbonate and a non-polar organic solvent, and performing a substitution reaction to obtain the formononetin derivative;
[0052] The formononetin has a structure shown in formula II, and the ruthenium complex has a structure shown in formula III:
[0053]
[0054] in, for
[0055]R is alkyl, substituted alkyl, alkoxy, hydroxy, carboxy, amino, alkenyl, alkynyl or phenyl.
[0056] In the present invention, unless otherwise specified, all preparation materials are commercially available products well known to those skilled in the art.
[0057] The invention mixes formononetin, ruthenium complex, potassium carbonate and non-polar organic solvent, and performs substitution reaction to obtain the formononetin derivative.
[0058] In the present invent...
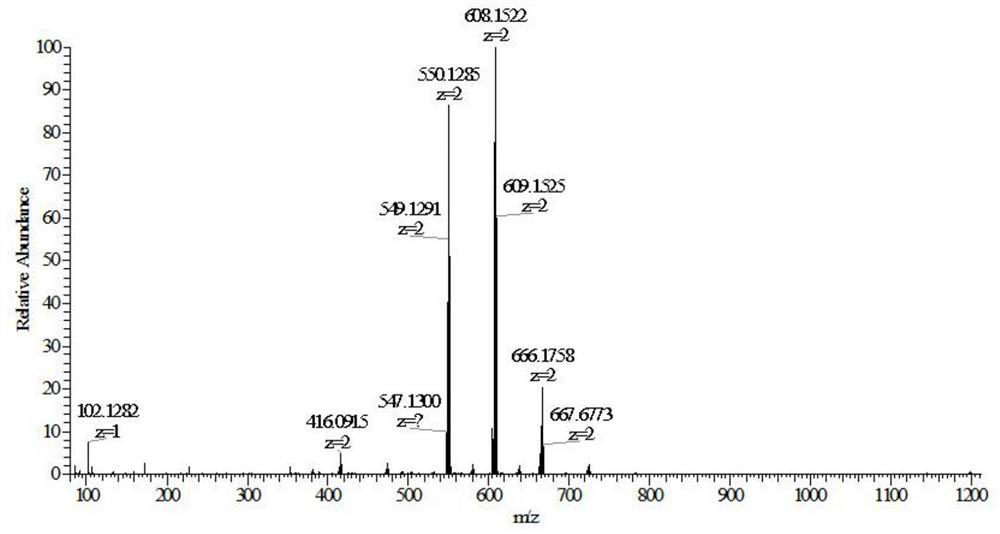
Embodiment 1
[0079] The reaction process of formononetin derivatives:
[0080]
[0081] Compound RBL083 (195 mg, 0.2 mmol), anhydrous potassium carbonate (2 g, 14.7 mmol), DMF 15 mL. Add it into a 50mL round bottom flask, heat at 60°C for 30min, add 1mL of 1,4-dibromobutane dropwise, react at 60°C for 24h, and the reaction is complete. Suction filtration under reduced pressure, the filtrate was diluted with 100 mL of distilled water, extracted three times with 100 mL of methyl tert-butyl ether, and the aqueous phase was collected. Add 200 mg of sodium perchlorate to the aqueous phase to form an orange suspension, which is filtered under reduced pressure. After the filter cake was dried, it was purified by alumina column chromatography, eluted with toluene: acetonitrile (1:1), and the first eluate (orange band) was collected as the compound RBL083-BBr, with a yield of 60%;
[0082] Add formononetin (26.8mg, 0.1mmol), ruthenium complex (RBL083-BBr, 111.2mg, 0.1mmol) successively in the ...
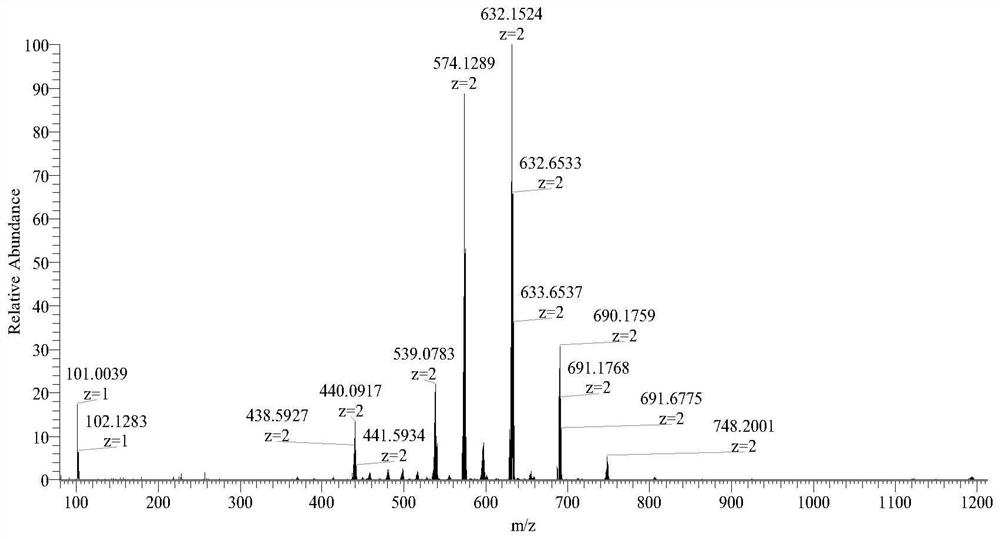
Embodiment 2
[0085] The reaction process of formononetin derivatives:
[0086]
[0087] Compound RPL083 (205 mg, 0.2 mmol), anhydrous potassium carbonate (2 g, 14.7 mmol), DMF 15 mL. Add it into a 50mL round bottom flask, heat at 60°C for 30min, add 1mL of 1,4-dibromobutane dropwise, react at 60°C for 24h, and the reaction is complete. Suction filtration under reduced pressure, the filtrate was diluted with 100 mL of distilled water, extracted three times with 100 mL of methyl tert-butyl ether, and the aqueous phase was collected. Add 200 mg of sodium perchlorate to the aqueous phase to form an orange suspension, which is filtered under reduced pressure. After the filter cake was dried, it was purified by alumina column chromatography, eluted with toluene: acetonitrile (1:1), and the first eluate (orange band) was collected as the compound RPL083-BBr, with a yield of 65%;
[0088] Add formononetin (26.8mg, 0.1mmol), ruthenium complex (RPL083-BBr, 116mg, 0.1mmol) successively in the mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com