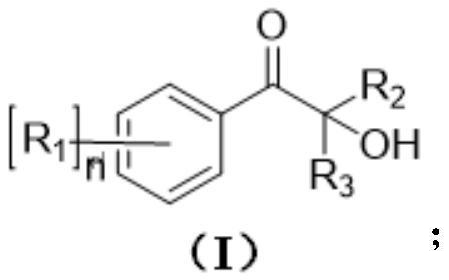
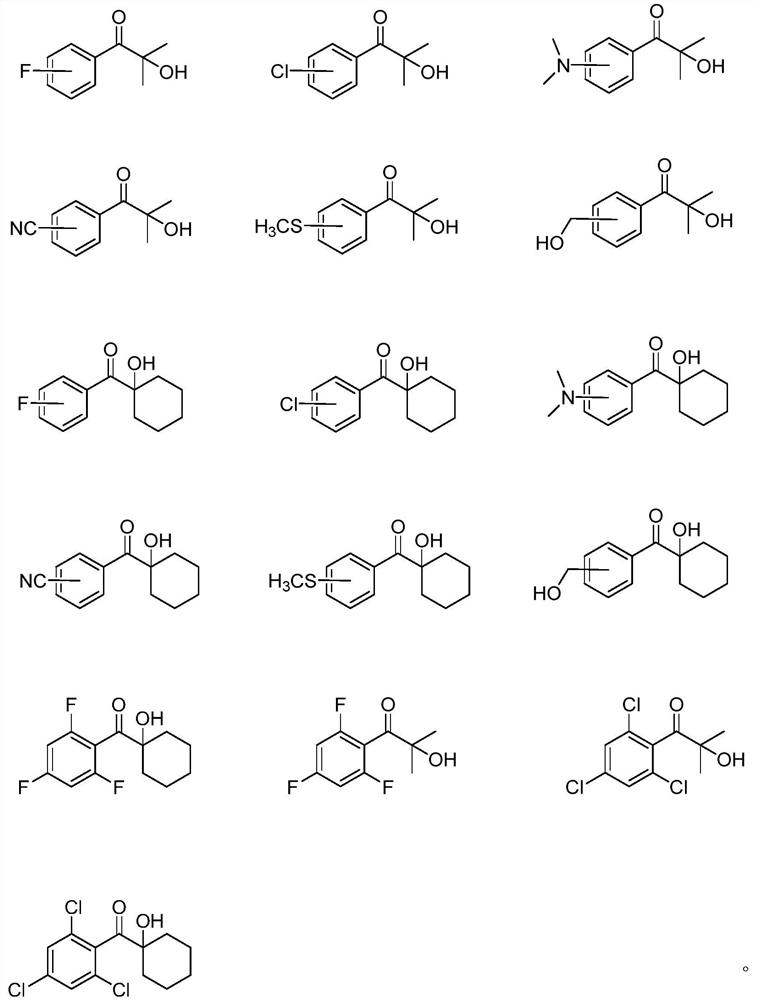
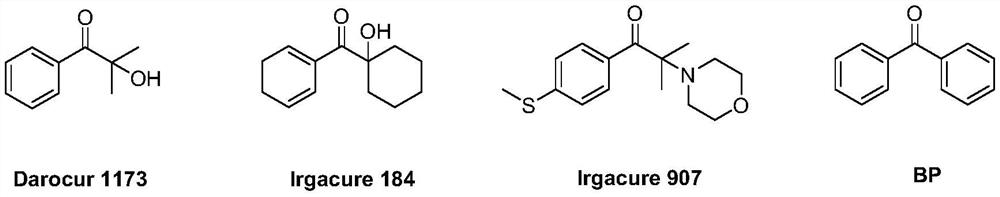
Monosubstituted and polysubstituted functional group aromatic ketone compounds, and preparation method and photopolymerization initiator thereof
A multi-functional group and photoinitiator technology, applied in the field of photopolymerization initiators and aromatic ketones, can solve the problems of reduced photopolymerization activity and high cost, and achieve the effect of reducing overall cost and improving photoinitiation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Preparation Example 1
[0047]
[0048] The specific operation steps are:
[0049] Under the protection of nitrogen, put 300 liters of anhydrous chlorobenzene into the reactor, start stirring and lower the temperature, put 130 kg of aluminum trichloride into the reactor, keep stirring and cooling for 1 hour, and then add isophthalic acid dropwise into the reactor. 100 kg of butyryl chloride was added dropwise for 2 hours and the temperature of the kettle was kept lower than 5° C. After the dropwise addition was completed, stirring was continued for 6 hours to obtain a Friedel-crafts acylation intermediate. Then, the positive and secondary chlorination tanks are alternately set up in two stages in series. First, the positive and secondary chlorination tanks are respectively put into 400 kg of Friedel-crafts acylation intermediates, and chlorine gas is introduced into the positive tank. After the chlorine gas is absorbed by the positive tank, the exhaust gas ...
Embodiment 2
[0051] Embodiment 2 Preparation Example 2
[0052]
[0053] Under the protection of nitrogen, put 300 liters of anhydrous chlorobenzene into the reactor, start stirring and lower the temperature, put 130 kg of aluminum trichloride into the reactor, keep stirring and cooling for 1 hour, and then add cyclic 135 kg of hexanoyl chloride, the time for the dropwise addition was 2 hours and the temperature of the kettle was kept lower than 5° C. After the dropwise addition, the stirring was continued for 6 hours to obtain the Friedel-crafts acylation intermediate. Then, the positive and secondary chlorination tanks are alternately set up in two stages in series. First, the positive and secondary chlorination tanks are respectively put into 400 kg of Friedel-crafts acylation intermediates, and chlorine gas is introduced into the positive tank. After the chlorine gas is absorbed by the positive tank, the exhaust gas containing chlorine Pass it into the auxiliary kettle for environme...
Embodiment 3
[0055] Example 3 Preparation Example 3
[0056]
[0057] Mix concentrated nitric acid and concentrated sulfuric acid to prepare a mixed solution, add trichloromethylbenzene into the reaction kettle, stir and add the mixed solution dropwise at a temperature of 50-55°C, and the dropping time is 50 -60min, after the dropwise addition, continue to stir for 15-20h to obtain the intermediate trichloro compound, dissolve 100 kg of the purified trichloro compound in dry tetrahydrofuran, and add dropwise to 10.5 Suspension of kilograms of magnesium chips and dry tetrahydrofuran, slowly add 27 kilograms of dry acetone dropwise after the reaction is completed, stir for 2 hours, add water and continue stirring for 30 minutes, collect the organic phase and obtain nitro-substituted aromatic ketones through vacuum distillation Product, yield 78%.
[0058] 1 H NMR (300 MHz, CDCl3), δ: 1.36 (6H, s), 8.34 (2H, dd), 8.42 (2H, dd).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com