Method for detecting afoxolaner intermediate by reversed-phase high performance liquid chromatography
A reversed-phase high-performance liquid phase and chromatographic detection technology, which is applied in the field of reversed-phase high-performance liquid chromatography to detect afolana intermediates, and achieves the effects of good chromatographic peak shape, good repeatability and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] S1: Preparation of the test solution: Weigh an appropriate amount of the test product of the intermediate of Afurana in a 20mL volumetric flask, dissolve it with a solvent and dilute to the mark, mix well, and prepare a sample containing 0.5mg of the intermediate per 1mL solution;
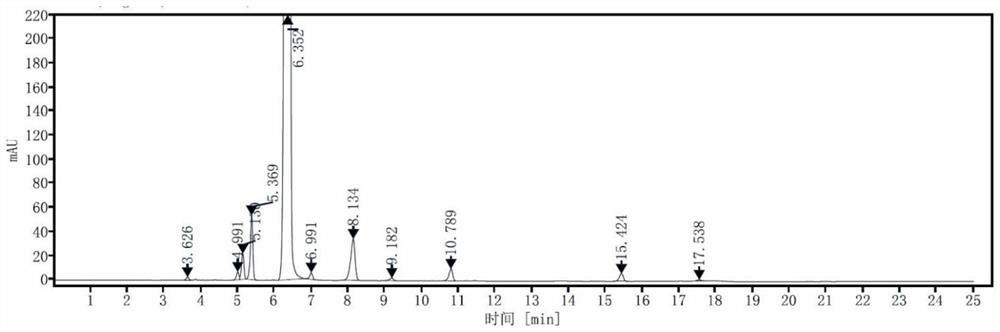
[0036] S2: Determination of reverse phase high performance liquid chromatography: YMC-Triart C18 chromatographic column: 4.6mm × 250mm, 5 μ m; mobile phase is acetonitrile: 0.01v / v% trifluoroacetic acid aqueous solution = (40 ~ 65): (60 ~ 35) (v / v) Gradient elution, gradient elution time and volume ratio of mobile phase acetonitrile: 0min~9min, 40%~55% operation; 9min~15min, 55%~65% operation; 15.1min~25min , 40% run. Flow rate: 1.0mL / min; Column temperature: 30°C; Detection wavelength of UV detector: 245nm; Sample volume: 10μL; figure 1 shown.
[0037] Depend on figure 1 It can be seen that the retention time of the main component in the test solution is 6.35min, the number of theoretic...
Embodiment 2
[0038] Embodiment 2: The influence of the volume fraction of trifluoroacetic acid aqueous solution on the detection of afolana intermediate by reversed-phase high-performance liquid chromatography
[0039] S1: according to embodiment 1 preparation need testing solution;
[0040] S2: In the chromatographic conditions of Example 1, keep acetonitrile unchanged, change the volume fraction of trifluoroacetic acid in the mobile phase B, and take the test sample for inspection. The results are shown in Table 1. Wherein, the degree of separation refers to the degree of separation between the afuraner intermediate and adjacent impurities.
[0041] Table 1
[0042] mobile phase B keep time Number of plates Tailing factor Separation Number of impurities 0.01v / v% trifluoroacetic acid in water 6.56min 21148 0.98 4.37 10 0.02v / v% trifluoroacetic acid aqueous solution 6.59min 26453 1.09 4.22 10 0.03v / v% trifluoroacetic acid aqueous solution 6...
Embodiment 3
[0044] Embodiment 3: the impact of column temperature on the detection of Afolana intermediates by reversed-phase high-performance liquid chromatography
[0045] S1: according to embodiment 1 preparation need testing solution;
[0046] S2: On the basis of the chromatographic conditions in Example 1, the column temperature was changed, and the sample solution of the test sample was taken for inspection. The results are shown in Table 2.
[0047] Table 2
[0048] Column temperature keep time Number of plates Tailing factor Separation Number of impurities 20℃ 7.09min 24252 0.97 4.42 8 25℃ 6.82min 27760 0.92 4.48 10 30℃ 6.56min 21148 0.98 4.37 10 35℃ 6.31min 26000 0.95 4.45 10 40℃ 6.09min 25114 0.96 4.28 10
[0049] The test results showed that if the temperature was too low, the detection of the intermediate of Afolana was affected, and the impurities could not be fully detected. When the column temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com