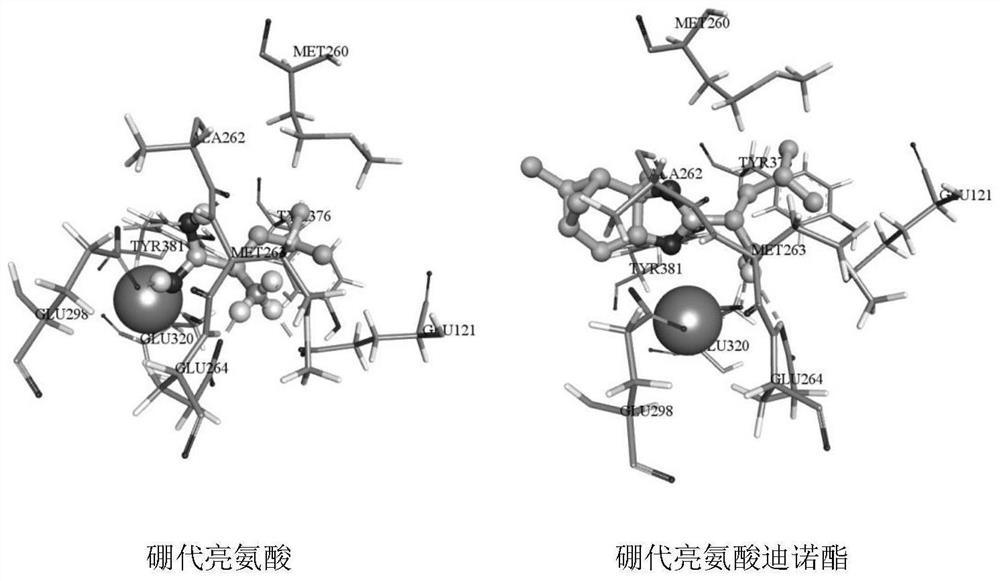
New application of boron-substituted leucine compound
A technique for substituting leucine and dinolate leucine, which is applied in the field of preparation of aminopeptidase N inhibitors, can solve the problem of weak proteasome inhibitory activity, insufficient tumor cell killing activity, citrate Instability and other problems, to achieve the effect of inhibiting tumor metastasis, tumor cell migration and invasion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The in vitro enzyme inhibitory activity experiment of embodiment 1 boronated leucine dinolate:
[0035] Use the method of preparing APN enzyme source (patent application number: 201810629864.8) for the evaluation of the inhibitory activity of boronoleucine and boronoleucine dinolate on aminopeptidase N, and Ubenimex positive control 1 , bortezomib served as positive control 2.
[0036] The test steps are as follows:
[0037] (1) K562-APN cells were sonicated to prepare cell homogenate, and seeded in 96-well plates;
[0038] (2) Adding compounds with different concentration gradients to the wells;
[0039] (3) Add APN substrate to 1.6mM after 5min and incubate for 1h;
[0040] (4) Measure the absorbance value at 405nm, and use Origin software to calculate IC50.
[0041] NOTE: The compounds used in the assay were boronoleucine, boronoleucine dinolate, ubenimex, or bortezomib.
[0042] The results showed that the IC50 value of boronoleucine on APN inhibitory activity ...
Embodiment 2
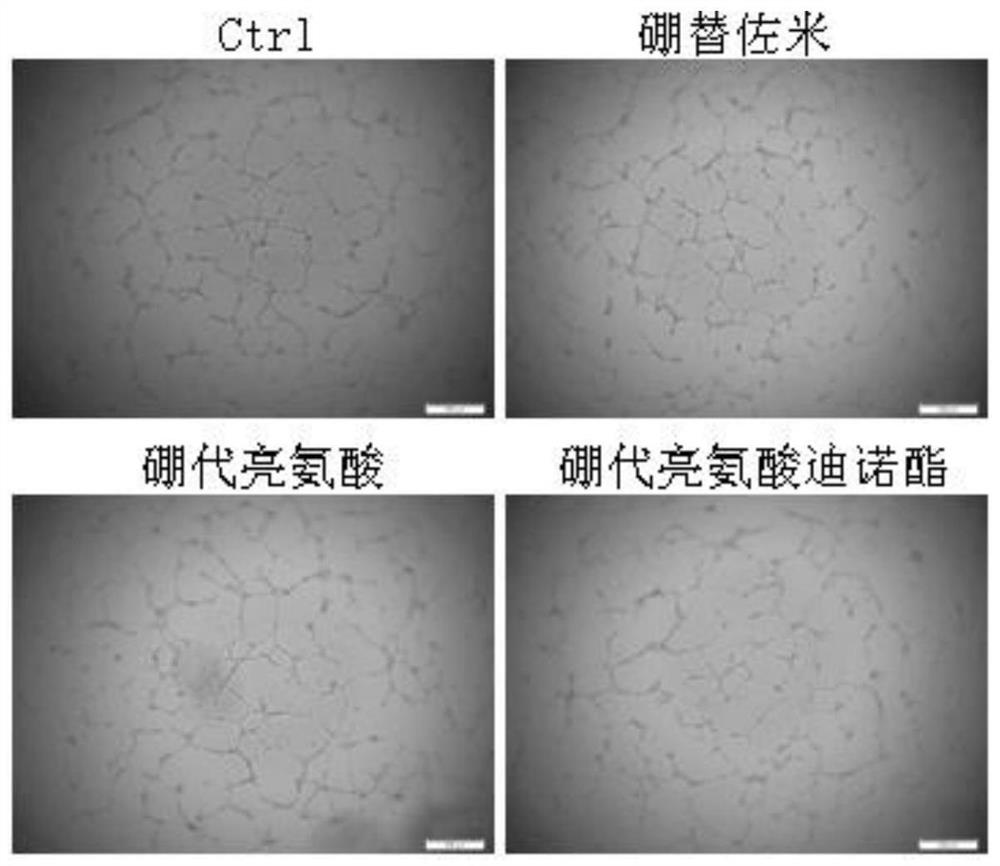
[0044] Example 2 Boroleucine and boroleucine dinolate inhibit the formation of two-dimensional tubes in HUVEC cells
[0045] Angiogenesis in tumor tissue can facilitate the metastasis of tumor cells. Using HUVEC cells to carry out two-dimensional tube formation test can reflect the ability of compounds to inhibit angiogenesis in tumor tissue. Boroleucine and Boleucine Dino Esters inhibit the ability of HUVEC cells to form two-dimensional tubes, and DMSO solvent is used as a blank control (denoted as Ctrl), and bortezomib is used as a positive control.
[0046] The test steps are as follows:
[0047] (1) Spread 50 μl of Matrigel gel diluted 1:1 in M199 medium on a 96-well plate, and incubate in an incubator;
[0048] (2) Add 100 μl of compound after 30 minutes, and make the concentration of the compound reach the working concentration after adding 50 μl of cells to the well;
[0049] (3) Treat cells, count, add 20000cell / 50μl / well to each well;
[0050] (4) After culturing f...
Embodiment 3
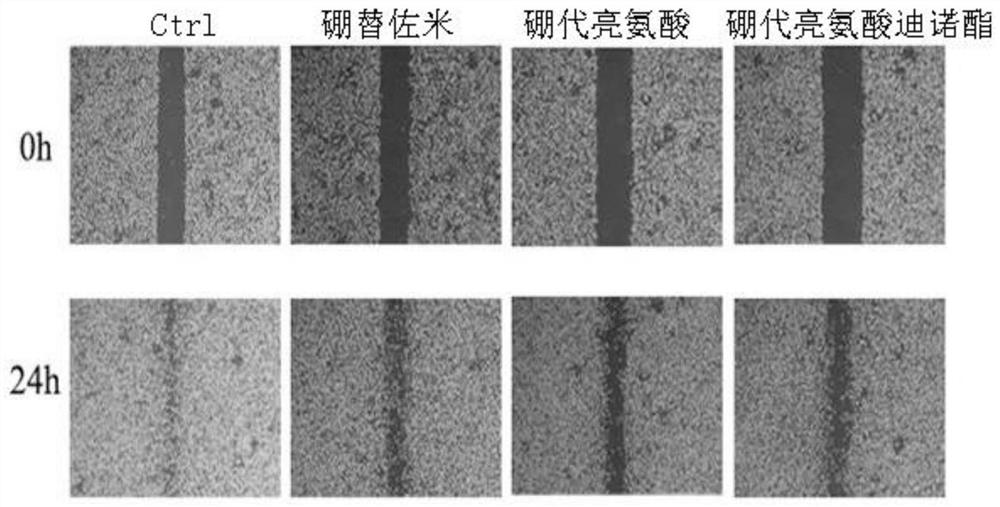
[0053] Example 3 Boroleucine and Boroleucine Dinoester Inhibit Breast Cancer Cell Migration Experiment
[0054] Scratch test can reflect the migration ability of breast cancer cells, investigate the ability of boronated leucine and boronated leucine dinolate to inhibit the migration of breast cancer cells, and use DMSO solvent as blank control (denoted as Ctrl), and boron Tezomib was used as a positive control.
[0055] The test steps are as follows:
[0056] (1) Lay breast cancer MDA-MB-231 cells to a 6-well plate, add 10 μM compound to treat for 2 days;
[0057] (2) With the aid of a pipette, draw a line with a white tip to make the line thickness consistent;
[0058] (3) Take pictures at 0 and 36 hours, and measure the migration distance by computer.
[0059] NOTE: The compounds used in the assay were boronoleucine, boronoleucine dinolate, DMSO solvent, or bortezomib.
[0060] The results show that if Figure 3-4 As shown, compared with the blank control and the positi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com