Single-layer hydrotalcite nano material and application thereof in efficient mineralization removal of high-concentration heavy metal ions in wastewater
A technology of heavy metal ions and nano-materials, which is applied in the field of mineralization treatment, can solve the problems of large consumption and weak stability, and achieve the effects of high-efficiency removal, large specific surface area, and improved adsorption rate and capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Preparation of single-layer MgAl-LDH nanomaterials:
[0040] 0.01875mol Mg(NO 3 ) 2 ·6H 2 O, 0.00625mol Al(NO 3 ) 3 9H 2 O plus 0.0.0625 molH 3 BO 3 Dissolve in 100mL to remove CO 2 water and recorded as solution A. 0.075mol NaOH dissolved in 100 mL to remove CO 2 water and recorded as solution B. where the excess base is used to neutralize the H 3 BO 3 Excess H carried + . Solution A and solution B were simultaneously and uniformly poured into a fully back-mixed rotary liquid film reactor with a rotating speed of 3000 rpm and back-mixed for 2 minutes to obtain a suspension.
[0041] Preparation of single-layer hydrotalcite peeling: the product after centrifugation of the suspension is peeled with acetone, and finally washed with ethanol until neutral, and then placed in a vacuum oven for drying.
[0042] Preparation of multilayer hydrotalcite: centrifuge the suspension, wash with deionized water, and dry in a vacuum oven.
[0043]2. Prepare the solut...
Embodiment 2
[0048] Preparation of single-layer NiFe-LDH material:
[0049] 0.01875mol Ni(NO 3 ) 2 ·6H 2 O, 0.00625mol Fe(NO 3 ) 3 9H 2 O plus 0.0.0625mol H 3 BO 3 Dissolve in 100mL to remove CO 2 water and recorded as solution A. Dissolve 0.075mol NaOH in 100mL to remove CO 2 water and recorded as solution B. where the excess base is used to neutralize the H 3 BO 3 Excess H carried + . Solution A and solution B were poured into a colloid mill with a rotating speed of 3000 rpm at a constant speed and mixed for 2 minutes to obtain a suspension.
[0050] Preparation of single-layer hydrotalcite peeling: the product after centrifugation of the suspension is peeled with acetone, and finally washed with ethanol to neutrality, then vacuum rotary evaporated and dried.
[0051] Preparation of multi-layer hydrotalcite: centrifuge the suspension, wash with deionized water, and dry by vacuum rotary evaporation.
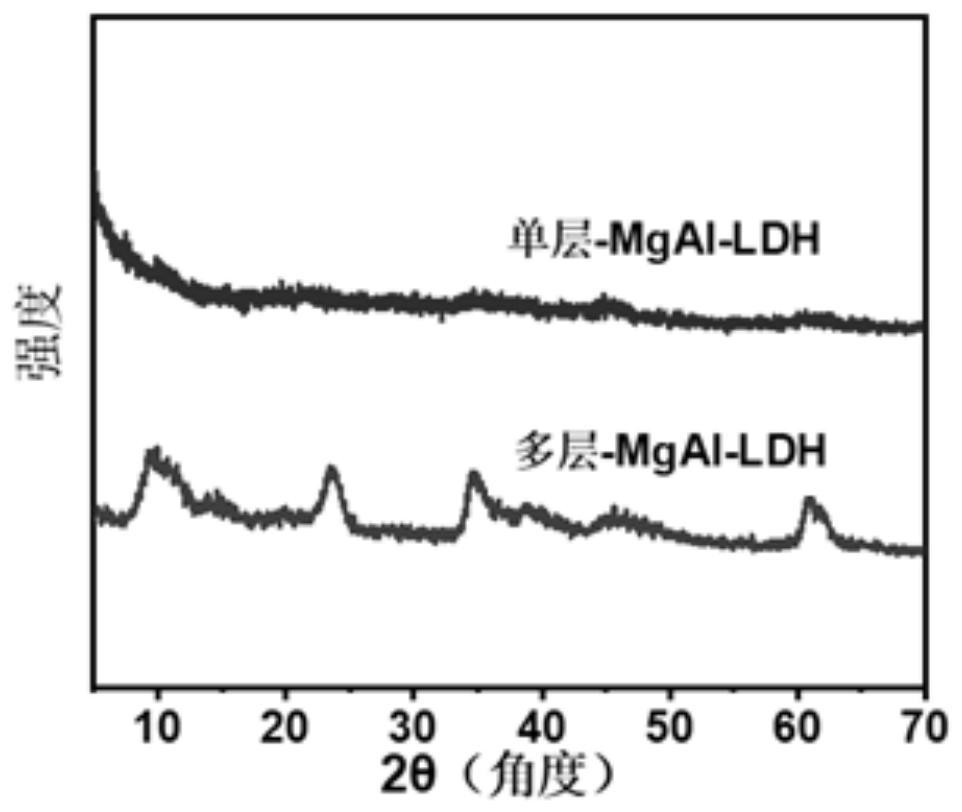
[0052] To characterize the material: e.g. Image 6 As shown, there is n...
Embodiment 3
[0054] Preparation of monolayer CoFe-LDH material:
[0055] 0.01875mol Co(NO 3 ) 2 ·6H 2 O, 0.00625mol Fe(NO 3 ) 3 9H 2 O plus 0.0.0625mol H 3 BO 3 Dissolve in 100mL to remove CO 2 water and recorded as solution A. Dissolve 0.075mol NaOH in 100mL to remove CO 2 water and recorded as solution B. where the excess base is used to neutralize the H 3 BO 3 Excess H carried + . Solution A and solution B were poured into a colloid mill with a rotating speed of 3000 rpm at a constant speed and mixed for 2 minutes to obtain a suspension.
[0056] Preparation of single-layer hydrotalcite peeling: the product after centrifugation of the suspension is peeled with acetone, and finally washed with ethanol until neutral, and then placed in a vacuum oven for drying.
[0057] Preparation of multilayer hydrotalcite: centrifuge the suspension, wash with deionized water, and dry in a vacuum oven.
[0058] Characterize the material: according to Figure 9 As shown, the monolayer Co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com