Patents
Literature
31 results about "Borate Boric Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Environment-friendly coated copper brazing filler metal
ActiveCN104907728AEfficient pre-joinReduce usageWelding/cutting media/materialsSoldering mediaAluminatePolyvinyl alcohol
The invention discloses environment-friendly coated copper brazing filler metal. The environment-friendly coated copper brazing filler metal comprises a copper brazing filler metal inner core and a copper brazing flux coating adhering to the outer side of the copper brazing filler metal inner core. The copper brazing flux coating comprises at least one of inorganic fluoride, potassium fluotitanate, anhydride, boric acid, borate, fluoroaluminate and elemental boron; the raw materials of copper brazing fluxes, polyvinyl alcohol, methylcellulose and water are prepared into a brazing flux solution according to a certain proportion, the surface of the copper brazing filler metal inner core is directly coated with the brazing flux solution, and the coated copper brazing filler metal can be obtained after the brazing flux solution is dried. The environment-friendly coated copper brazing filler metal has the advantages that the production process is simple, the production cost is low, the finished brazing filler metal is environmentally friendly, free of pollution and convenient to use, and the coating bonding strength is high; the brazing fluxes can be pre-added conveniently, quickly, quantitatively and efficiently, and the use amount of the brazing fluxes is effectively reduced; since the coated copper brazing filler metal does not contain organic bonding agents, the coated copper brazing filler metal meets the ROHS standard, pollution to environment during brazing and burning is greatly reduced, body health of operators is protected, and the labor intensity of the operators in the brazing process is greatly relieved.
Owner:CHINA INNOVATION ACADEMY OF INTELLIGENT EQUIP CO LTD
Method for preparing hard carbon negative electrode materials of lithium ion battery
The invention discloses a method for hard carbon negative electrode materials of a lithium ion battery and belongs to the technical field of hard carbon negative electrode materials of the lithium ion battery. By means of the method, one or several kinds with any proportion of sulfuric acid, sulfate, boric acid, borate, phosphoric acid, phosphate, muriatic acid, muriate and ammonia or ammonium salt is / are chosen as a catalyst / catalysts for preparing the hard carbon negative electrode materials of the lithium ion battery in a catalysis method, and compared with the catalysts used in the prior art, the catalysts used in the preparing process of the materials are reduced in low-temperature section stabilized heat treatment temperature, the time is shortened by nearly 10 times, a large number of resources are saved, production cost is reduced, and capacity is improved.
Owner:DONGFANG ELECTRIC CORP LTD
Method for preparing high-purity lactulose
ActiveCN102020680AImprove conversion rateHigh yieldSugar derivativesDisaccharidesChromatographic separationIon exchange
The invention relates to the field of the preparation of functional sugar, and relates to a method for preparing high-purity lactulose. The method for preparing the high-purity lactulose comprises the following steps of: preparing lactose solution at the concentration of 15 to 20 percent; adding a boric acid and sodium hydroxide (NaOH) into the solution, and adjusting a reaction to obtain conversion liquid; performing ion exchange sodium ion (Na<+>)-removing treatment on the conversion liquid; decoloring a granular active carbon column; removing the boric acid from the lactose solution to obtain a lactose solution dry substance with the boric acid content of less than 1 mg / kg; concentrating the lactose solution until the concentration is 50 to 60 percent; separating by using a 10-bed continuous chromatographic separation device; and performing aqueous phase crystallization, wherein the cooling speeds are 0.8 DEG C per hour at the temperature of between 60 and 50 DEG C, 1 DEG C per hour at the temperature of between 50 and 30 DEG C and 1.1 DEG C per hour at the temperature of between 30 and 13 DEG C. The conversion rate of the lactulose can be increased, so that the yield of the lactulose products is greatly improved; granular active carbon is decolored; moving bed chromatographic separation is continuously simulated, so continuous chromatographic separation of the high-purity lactulose can be realized, the purity of the lactulose reaches over 92 percent so as to facilitate aqueous phase crystallization; and the purity can reach over 98 percent due to the aqueous phase crystallization.
Owner:BAOLINGBAO BIOLOGY
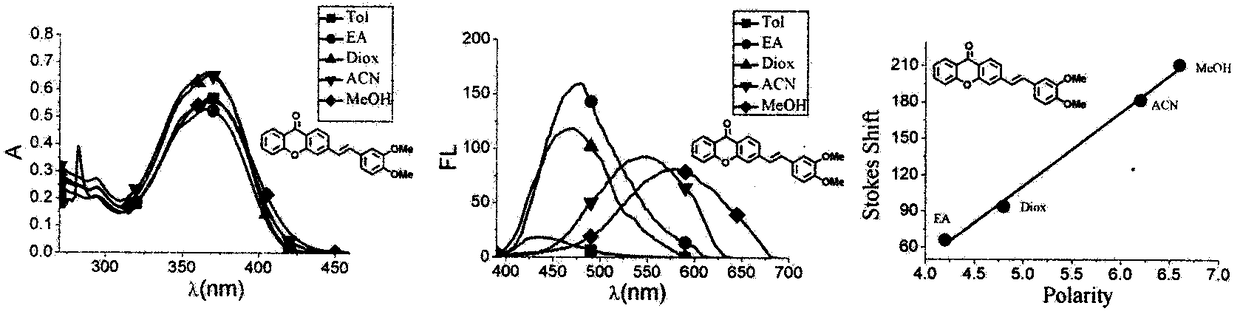
Xanthone fluorochrome and application
InactiveCN108864058AEasy to synthesizeMethine/polymethine dyesMicrobiological testing/measurementArylAnthracene
The invention provides xanthone fluorochrome and application therefore to design synthesis of a fluorescence probe. The Xanthone fluorochrome has the following structure general formulas I, II, III and IV shown in the description, wherein R1, R2, R3 and R4 are respectively selected from hydrogen, halogen, hydroxyl, NH2, sulfydryl, cyano-group, nitryl, N,N-bis-C1-4 alkyl amino group, aldehyde group, carboxyl, amido, sulfoamido, C5-10 aryl, C2-8 heterocyclic aryl, C1-6 alkyl, boric acid, boronic acid pinacol ester and OR (wherein R is C1-4 saturated alkyls or unsaturated alkyls).
Owner:成都必凯科技有限公司
Use of polycarboxylate ethers in combination with other additives for milling cement
InactiveUS20160024307A1Improve early strengthReducing floating of rustInksCement productionIron sulfateAlkaline earth metal
Use of an aqueous composition containing at least one polycarboxylate ether as cement grinding aid, wherein the aqueous composition contains one or more additives, or the aqueous composition is used in combination with one or more additives, and wherein the additive is selected from 1,3-propanediol, a carboxylic acid, a sulfonated amino alcohol, boric acid, a salt of boric acid, a salt of phosphoric acid, sorbitol, a saccharide, a gluconate, iron sulfate, tin sulfate, an antimony salt, an alkali salt, an alkaline earth salt, lignin sulfonate, glycerol, melamine, melamine sulfonate and mixtures thereof.
Owner:SIKA TECH AG
Photocatalytic coating
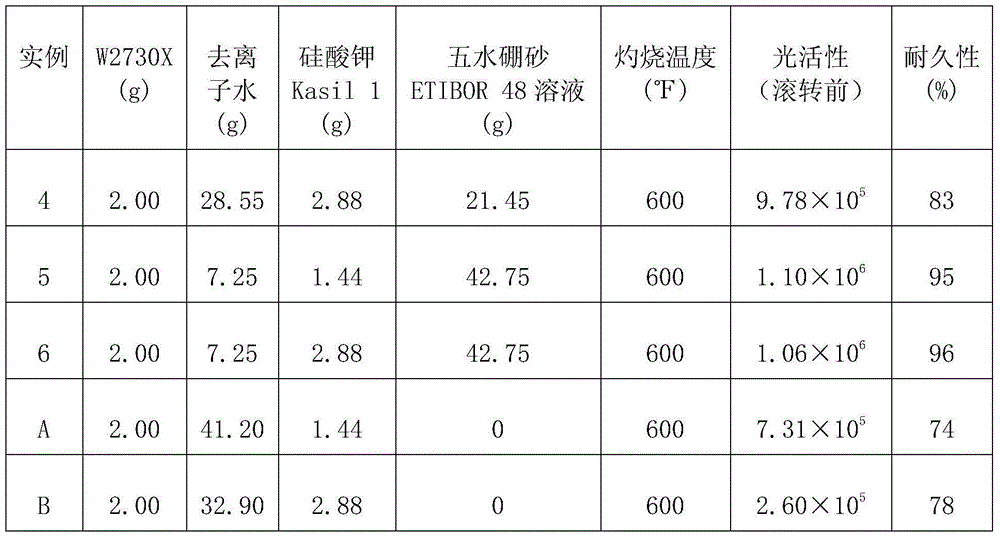
In one aspect, the present invention is directed to a coating composition. The coating composition comprises photocatalytic particles and an alkali metal silicate binder comprising a boric acid, borate, or combination thereof. In another aspect, the present invention is directed to a coated article. The coated article has a photocatalytic coating with improved durability on its external surface that is formed from the aforethe coating composition.
Owner:3M INNOVATIVE PROPERTIES CO
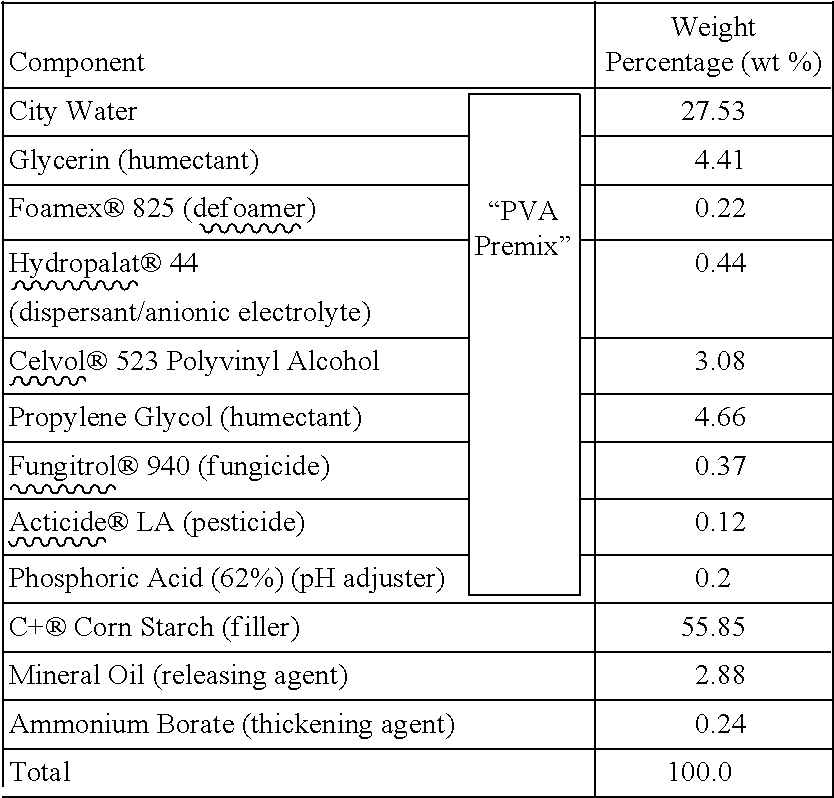
Moldable compositions and methods of using the same
The present invention provides moldable compositions that are capable of drying and hardening if left in open air at room temperature for a period of time. Embodiments of the moldable compositions comprise water, at least one polar polymeric resin (e.g., polyvinyl alcohol), at least one thickening agent (e.g., boric acid, borate salt, or hydrate of a borate salt), at least one humectant (e.g., glycerin, propylene glycol, etc.), at least one filler (e.g., corn starch, tapioca, or arrowroot), optionally at least one releasing agent (e.g., mineral oil) and optionally at least one additive.
Owner:CRAYOLA
Low-temperature kiln-transformed zirconium white glaze without bottom glaze, its preparation and application method
The invention belongs to the field of ceramic production and particularly relates to a ground-glaze-free low-temperature transmutation zirconium white glaze formula, a preparation method and a use method thereof. The ground-glaze-free low-temperature transmutation zirconium white glaze formula includes feldspar powder, boric acid, barium borate, limestone, zinc oxide, lead oxide, lead silicate and bentonite. The glaze is low in cost, is convenient to use, is low in sintering temperature and has a unique artistic feel. Glaze surface produced by sintering the ground-glaze-free low-temperature transmutation zirconium white glaze has a zirconium white dark light bottom color, wherein irregular black spots are uniformly distributed on the glaze surface. The size and distribution density of the black spots are changed according to the model of a ceramic product and glaze thickness. The glaze surface has a stone-like appearance and a slight flowing-tendency effect, thereby achieving unique artistic effects. Porcelain produced with the glaze is increased in grade and market price and is high in economic value. The glaze can be produced in large scale, can be popularized quickly and is simple in use, thereby achieving strong market competitiveness.
Owner:FUJIAN PROVINCE DEHUA COUNTY GRANGTOP CERAMICS CO LTD
Qualitative and quantitative marker for bombyx batryticatus counterfeit products and detection method thereof
ActiveCN112730291AGood precisionImprove stabilityColor/spectral properties measurementsAgainst vector-borne diseasesMedicineBoronic acid
The invention discloses a qualitative and quantitative marker for bombyx batryticatus counterfeit products and a detection method thereof. The marker is borax or boric acid. According to the invention, borax in the bombyx batryticatus counterfeit product is rapidly enriched and subjected to microwave extraction through an ethyl hexanediol n-butyl alcohol solution, borax is converted into boric acid under the acidic condition, boric acid and curcumin protonated by sulfuric acid can be subjected to a chromogenic reaction, and the content of borax in the bombyx batryticatus counterfeit product can be qualitatively and quantitatively determined through a colorimetric method. According to the method, the authenticity of bombyx batryticatus can be identified through qualitative and quantitative detection of borax in counterfeit bombyx batryticatus. The method has great application prospects and social value in stiff silkworm variety authenticity identification application. The method provided by the invention selects n-butyl alcohol with low toxicity as an extraction solvent, is safe to operate, is suitable for large-scale popularization and application, has the characteristics of rapid, low-toxicity, stable, qualitative and quantitative detection of borax in bombyx batryticatus counterfeit products, and provides technical support for quality standardization and normalization of traditional Chinese medicinal materials bombyx batryticatus and clinical medication safety.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM +1
Photocatalytic coating
In one aspect, the present invention is directed to a coating composition. The coating composition comprises photocatalytic particles and an alkali metal silicate binder comprising a boric acid, borate, or combination thereof. In another aspect, the present invention is directed to a coated article. The coated article has a photocatalytic coating with improved durability on its external surface that is formed from the aforesaid coating composition.
Owner:3M INNOVATIVE PROPERTIES CO
Formation liquid, formation method and anode foil
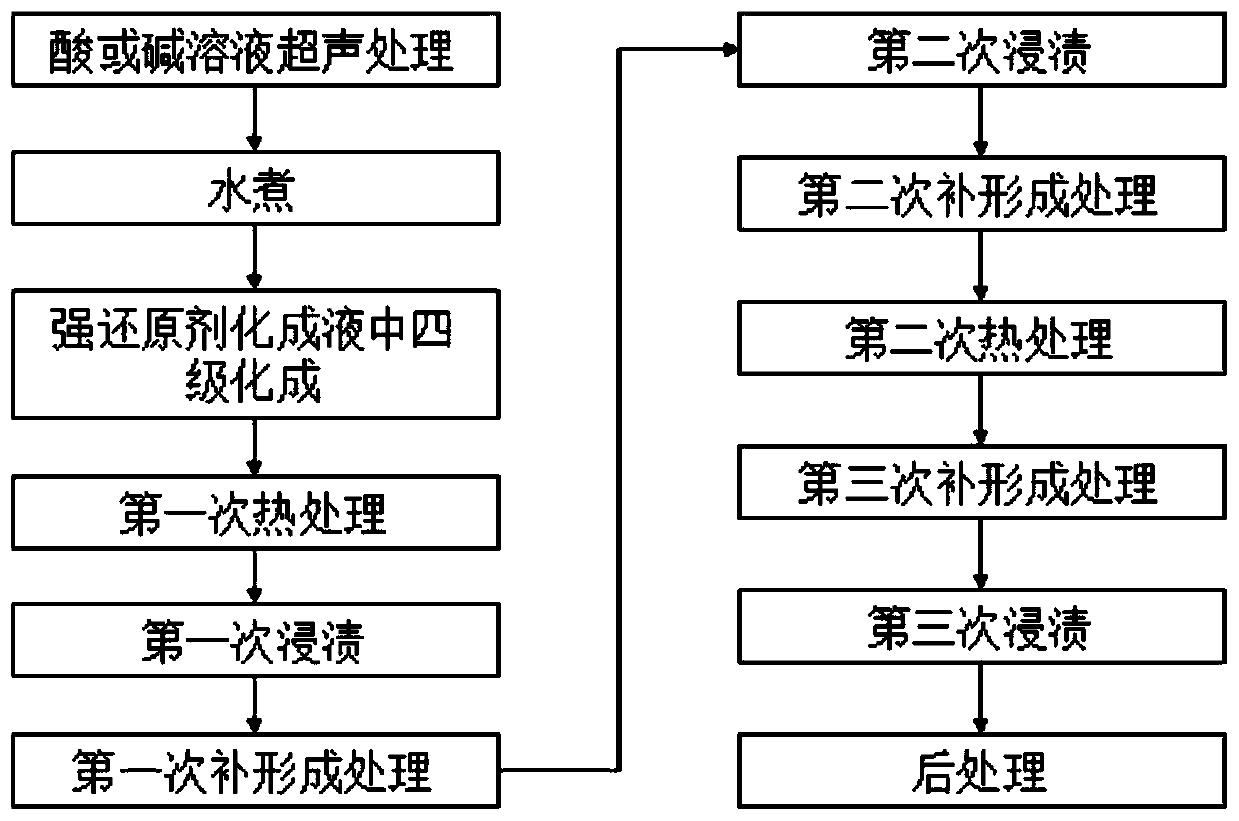
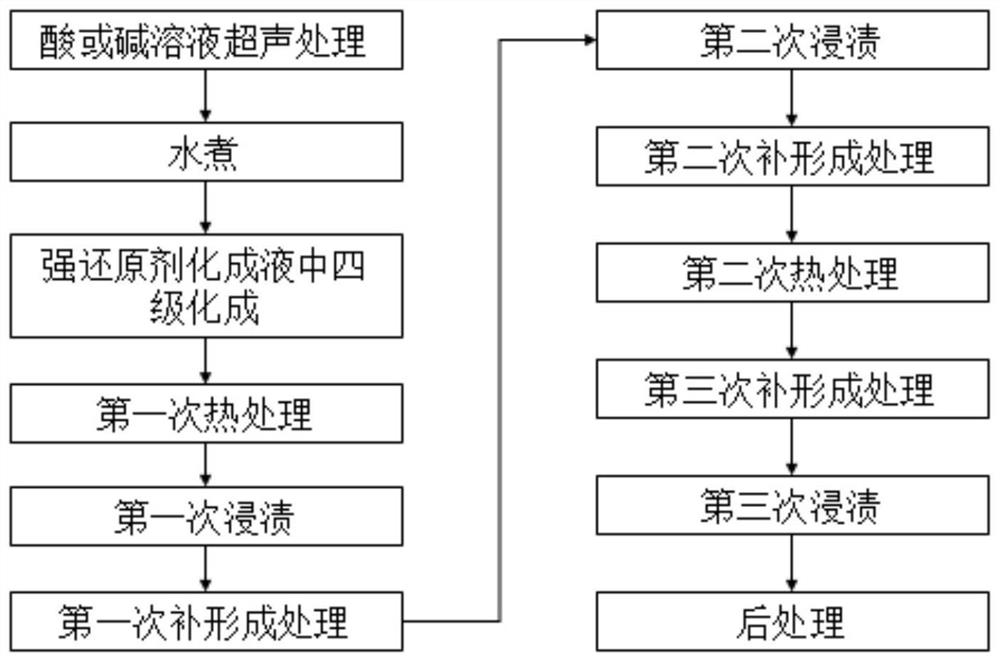
ActiveCN111139508AImprove bindingImprove specific volume reductionAnodisationElectrolytic capacitorsAmmonium borateCapacitor
The invention provides laminated foil formation liquid, a formation method, and anode foil obtained by the formation method. The formation method comprises the following steps: pretreatment; boiling with water; four-stage formation in a strong reducing agent formation solution; first heat treatment; first dipping; first complementary formation treatment; second dipping; secondary complementary formation treatment; secondary heat treatment; third complementary formation treatment; third dipping; and post-processing to obtain the formed laminated foil, namely the anode foil. The strong reducingagent formation solution is an aqueous solution containing 2-10 wt% of boric acid, 0.01-1 wt% of ammonium borate and 0.001-10 wt% of strong reducing agents. The specific volume of the anode foil obtained by formation using the formation solution is high, and the anode foil is suitable for being used for electrode materials of aluminum electrolytic capacitors.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
Environmentally friendly sheathed copper solder
ActiveCN104907728BEfficient pre-joinReduce usageWelding/cutting media/materialsSoldering mediaCelluloseAluminate
The invention discloses an environment-friendly sheathed copper brazing filler metal, which comprises a copper brazing filler metal inner core and a copper brazing flux coating adhering thereto, and the raw materials of the copper brazing flux coating include inorganic fluoride, potassium fluoroborate, boric anhydride , boric acid, borate, fluoroaluminate, elemental boron at least one; after the brazing flux raw material, polyvinyl alcohol, methyl cellulose and water are formulated into a flux liquid according to a certain ratio, it is directly coated On the surface of the inner core of the brazing filler metal, the coated brazing filler metal can be obtained after drying. The invention has the advantages of simple production process, low production cost, environmental protection and pollution-free finished solder, convenient use, high bonding strength of coating, etc., realizes convenient, fast, quantitative and efficient pre-addition of flux, effectively reduces flux The amount of usage; because it does not contain organic binders and complies with ROHS standards, the pollution to the environment is greatly reduced during brazing and burning, thereby protecting the health of operators and greatly reducing the labor intensity of the brazing process.
Owner:CHINA INNOVATION ACADEMY OF INTELLIGENT EQUIP CO LTD
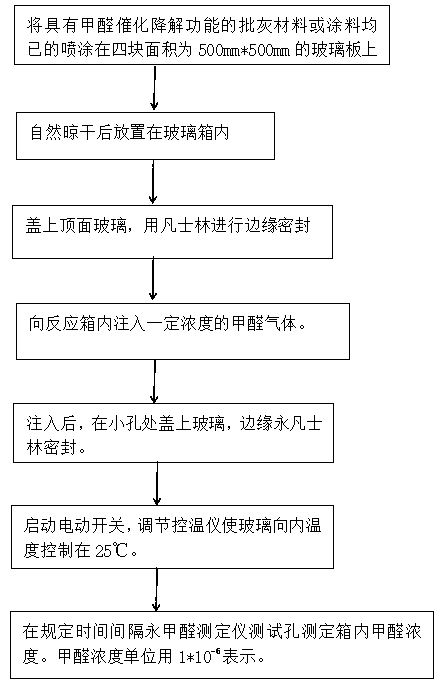
Puttying material with catalytic formaldehyde degradation and absorption function, and preparation method thereof
ActiveCN110975596APromote absorptionOrganic-compounds/hydrides/coordination-complexes catalystsDispersed particle separationSodium bicarbonatePolyvinyl alcohol
The invention discloses a puttying material with catalytic formaldehyde degradation and absorption function, and a preparation method thereof, wherein the puttying material comprises the following components by weight: 30-40 parts of inorganic powder, 5-10 parts of gypsum, 1-5 parts of anatase-shaped titanium dioxide powder, 20-40 parts of polyvinyl alcohol, 40-60 parts of water and 10-20 parts ofa cross-linking agent, wherein the acid-alkali regulator is one or a plurality of materials selected from hydrochloric acid, sodium dihydrogen phosphate, potassium dihydrogen phosphate, sodium carbonate and sodium bicarbonate, and the cross-linking agent is at least one selected from boric acid, borate, glutaraldehyde and citric acid. The preparation method comprises the following steps: 1, weighing polyvinyl alcohol and water according to a weight part ratio, and heating the water to 90 DEG C to fully dissolve the polyvinyl alcohol; 2, adjusting the pH value of the polyvinyl alcohol aqueoussolution obtained in the step 1 to 2-3, uniformly mixing the polyvinyl alcohol aqueous solution and a cross-linking agent, and standing for 24-48 hours to obtain a cross-linked polyvinyl alcohol aqueous solution; 3, adjusting the pH value of the cross-linked polyvinyl alcohol aqueous solution obtained in the step 2 to 7-8; and 4, uniformly mixing the cross-linked polyvinyl alcohol aqueous solutionobtained in the step 3, inorganic powder, gypsum and anatase-shaped titanium dioxide powder to obtain the puttying material with the function of catalytic degradation of formaldehyde.
Owner:南京冰锋环保科技有限公司
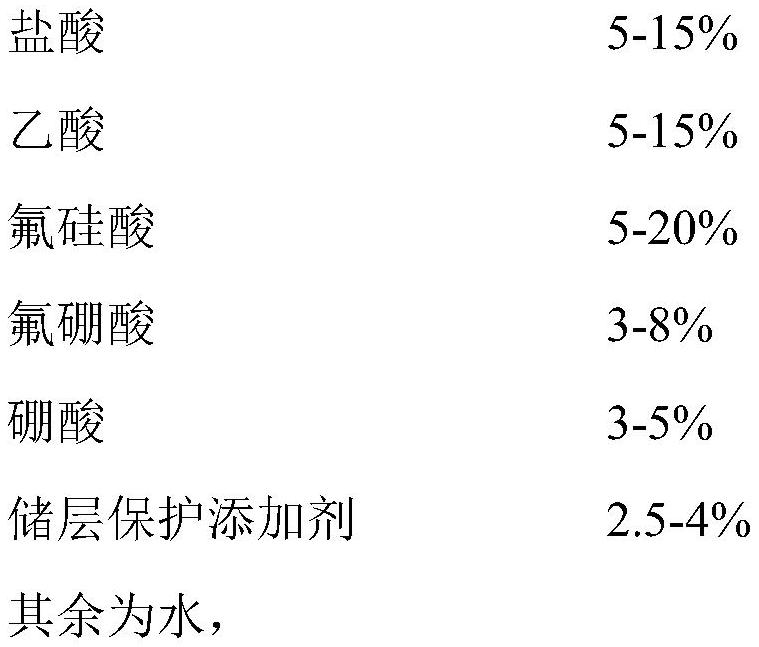
Porous sandstone reservoir permeability-enhancing sand-stabilizing acidizing compound working solution as well as preparation method and application thereof
PendingCN114591721AImprove permeabilityIncrease production capacityFluid removalDrilling compositionFluoboric acidSilicon dioxide
The invention provides a loose sandstone reservoir permeability-enhancing sand-stabilizing acidification composite working solution and a preparation method and application thereof. The loose sandstone reservoir permeability-enhancing sand-stabilizing acidification composite working solution is composed of hydrochloric acid, acetic acid, fluosilicic acid, fluoboric acid, boric acid, a reservoir protection additive and water. The compound working fluid can be used for deeply and slowly acidizing to increase the permeability of a loose sandstone reservoir and improve the productivity of an oil-water well; fluorosilicic acid is adopted as a main acid, can react with albite, potassium feldspar, kaolinite and montmorillonite in a reservoir stratum under the combined action of hydrochloric acid and acetic acid, and basically does not react with a silicon dioxide skeleton in quartz, and boric acid is adopted to absorb fluosilicic acid and fluoboric acid to generate hydrofluoric acid, so that a sandstone skeleton is protected and prevented from being damaged by acidification, and thus sand production blockage is aggravated; boric acid and fluoboric acid synergistically repair and loosen the sandstone reservoir; and secondary precipitation of fluosilicate except calcium fluoride is prevented under the combined action of boric acid, hydrochloric acid and acetic acid.
Owner:CNOOC ENERGY TECH & SERVICES
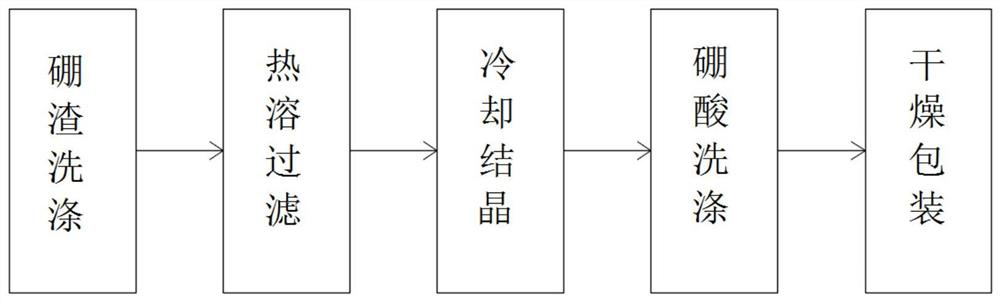
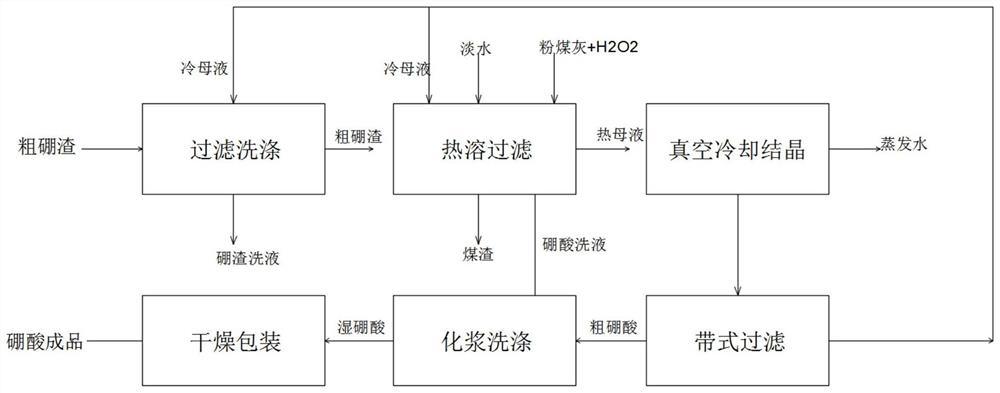
Preparation process of boric acid
PendingCN113860322ASimple preparation processReduce manufacturing costBoron oxyacidsChemical industryFluid phase
The invention relates to the technical field of chemical industry, and discloses a production process of boric acid. The production process comprises the following steps: S1, performing boron slag washing: adding a saturated boric acid mother liquor into coarse boron slag, enabling part of magnesium chloride, magnesium sulfate and other impurities to enter a liquid phase, and separating part of the impurities; S2, performing hot melting and filtering: adding freshwater and part of the saturated boric acid mother liquor into the washed coarse boron slag, and heating to enable all boric acid to enter the liquid phase; S3, performing cooling crystallization: cooling the hot boric acid mother liquor to normal temperature through a vacuum crystallizer, separating a solid phase from the mother liquor by boric acid, and separating to obtain crude boric acid; S4, performing boric acid washing: adding pure water into the crude boric acid, enabling a small amount of magnesium chloride, magnesium sulfate and other impurities entrained in the crude boric acid to enter the liquid phase, and obtaining wet boric acid; and S5, drying and packaging: dehydrating the wet boric acid through steam heating, and packaging to obtain a boric acid product. The preparation process of boric acid is simple, low in preparation cost, few in by-products and high in yield, and the prepared boric acid is high in purity.
Owner:河北昊德生物科技有限公司
Cleaning formulations
ActiveUS10626353B2Inorganic/elemental detergent compounding agentsOrganic detergent compounding agentsAlcoholBoronic acid
Owner:FUJIFILM ELECTRONICS MATERIALS US
Preparation method of 2-aminothiazole
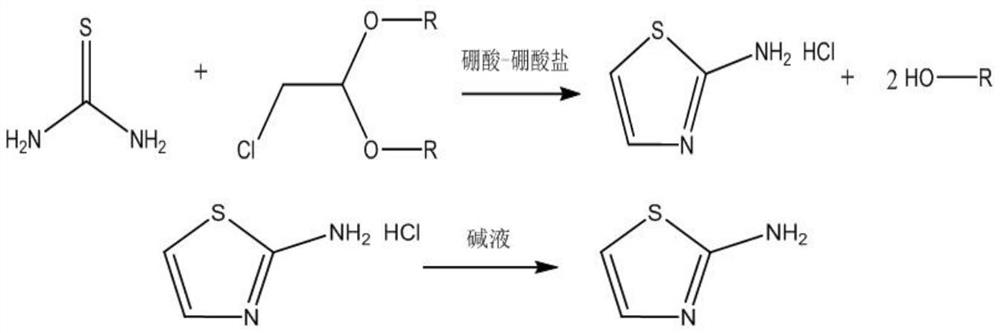
ActiveCN114853692AAvoid decompositionReduce generationOrganic chemistryChemical recyclingPtru catalystThiourea
The invention discloses a preparation method of 2-aminothiazole, and belongs to the field of fine chemical engineering. The preparation method of the 2-aminothiazole comprises the following steps: S1, fully reacting thiourea, chloroacetaldehyde acetal and a boric acid-borate composite catalyst at 90-110 DEG C by a solvent-free one-pot method to obtain a reaction product; and S2, removing by-products in the reaction product, adjusting the pH value to 9-10, separating out at 0-10 DEG C, filtering to obtain crystals, and purifying and drying the crystals to obtain the 2-aminothiazole. According to the method, thiourea and chloroacetaldehyde acetal which cannot be subjected to mutual dissolution reaction can be subjected to out-phase reaction to generate 2-aminothiazole under the solvent-free condition by adopting the boric acid-borate composite catalyst, the product yield and purity are improved, the yield can be improved to 95% or above from 75%-80% in the prior art, and the purity is improved to 97%-99% from 80%-90%. In addition, color change and even deterioration of the 2-aminothiazole are avoided through specific pH control, and white 2-aminothiazole can be obtained.
Owner:INST OF CHEM ENG GUANGDONG ACAD OF SCI
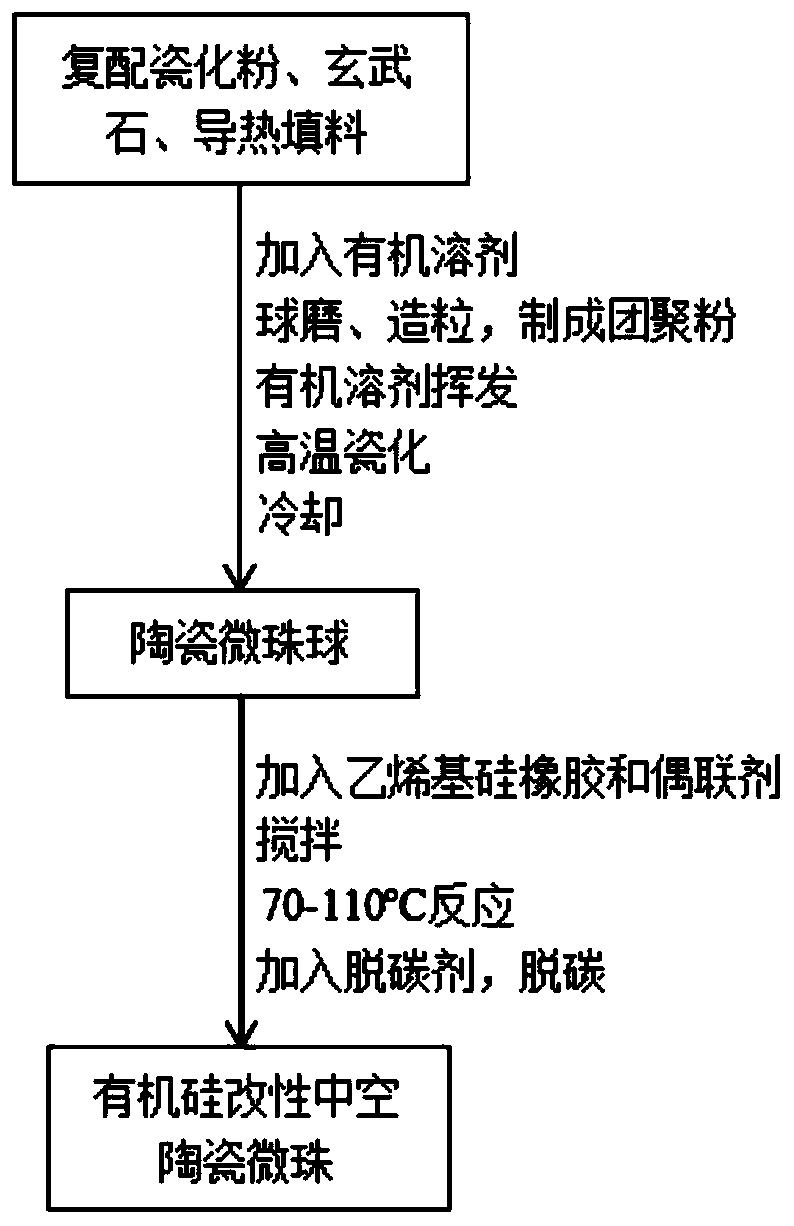
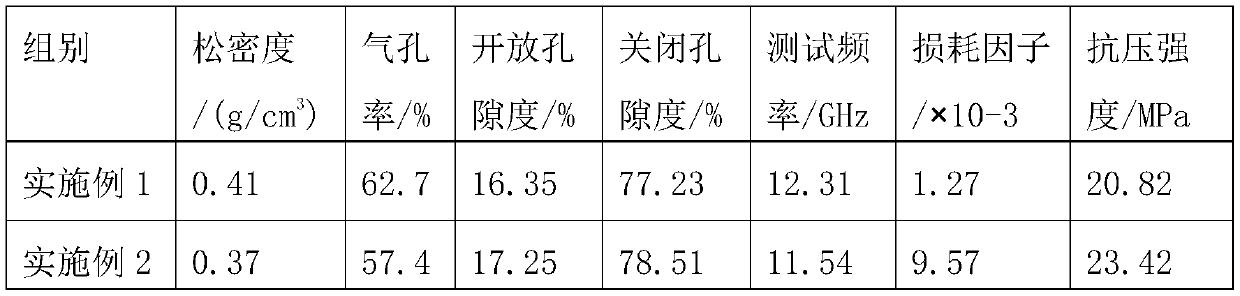
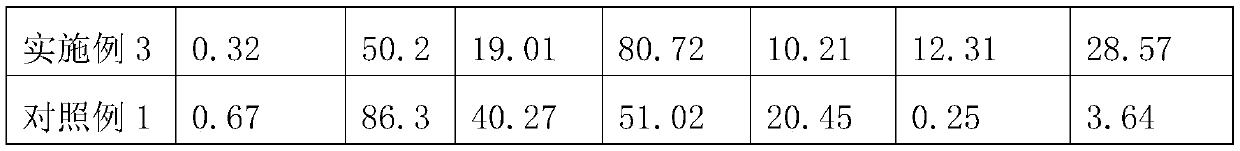
A kind of organosilicon modified hollow ceramic microbead and its preparation and application
ActiveCN108727058BReduce interfacial energyIntensity effectCeramicwareClaywaresBoron nitrideSilicone rubber
Owner:江苏省苏安能节能建材科技有限公司
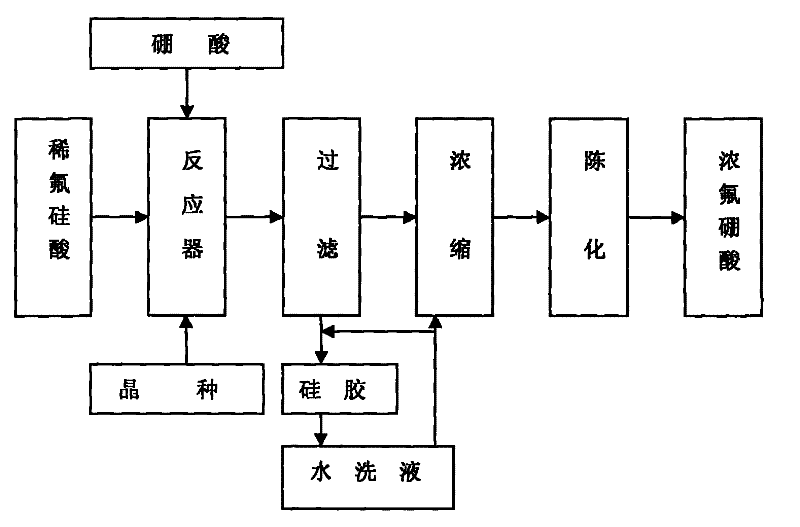
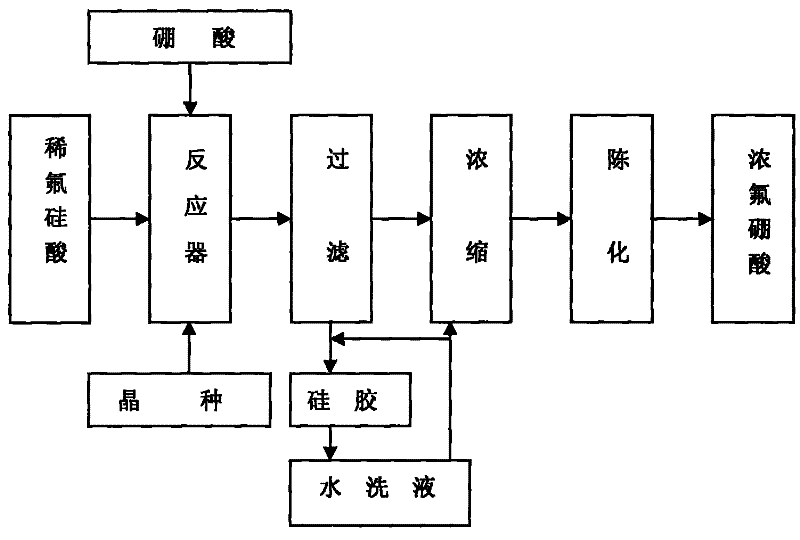
Method for preparing fluoroboric acid using by-product fluosilicic acid with low concentration in wet method phosphoric acid production process
ActiveCN101497448BReduce the feasible concentrationAvoid decompositionSolid waste disposalBoron halogen compoundsPhosphorous acidO-Phosphoric Acid
The invention discloses a method for preparing fluoboric acid from a byproduct, namely low-concentration hydrofluosilicic acid of phosphorous acid by a wet process and preparation of the fluoboric acid, and in particular relates to the method for preparing the fluoboric acid by inducing the crystallization by the byproduct, namely the low-concentration hydrofluosilicic acid of the phosphorous acid by the wet process. The method comprises the following steps that: a, the low-concentration hydrofluosilicic acid is sent into a reactor, silicon dioxide is added into the reactor to serve as an inoculating seed, and the stirring is performed; b, a suspension of the hydrofluosilicic acid and the silicon dioxide is preheated to a temperature which is more than 50 DEG C; c, solid boric acid is slowly added into the hydrofluosilicic acid, the ratio of the actual use level to the theoretical use level of the boric acid is between 0.95 and 1.05, and the adding time is between 60 and 70min; d, themixture reacts for 2 to 3h under the condition that the temperature is between 80 and 90 DEG C and the stirring speed is between 50 and 60r / min; e, the filtration is performed immediately after the reaction is stopped; and f, a filtrate is concentrated to a concentration which is more than or equal to 40 percent under the conditions of a temperature of between 50 and 80 DEG C, the pressure of between -0.06 and -0.07MPa, and the like, and is aged for 24 hours to obtain a finished product. The method reduces the feasible concentration of the hydrofluosilicic acid, ensures that dilute acid directly takes part in the reaction without being concentrated, shortens a process route, also avoids the decomposition of the hydrofluosilicic acid during the concentration, and improves the recovery rateof fluorine.
Owner:YUNNAN YUNTIANHUA
Novel negative electrode material of sodium ion battery as well as preparation and application thereof
ActiveCN111422880ASave energyHigh purityNegative electrodesSecondary cellsIron sulfateElectrical battery
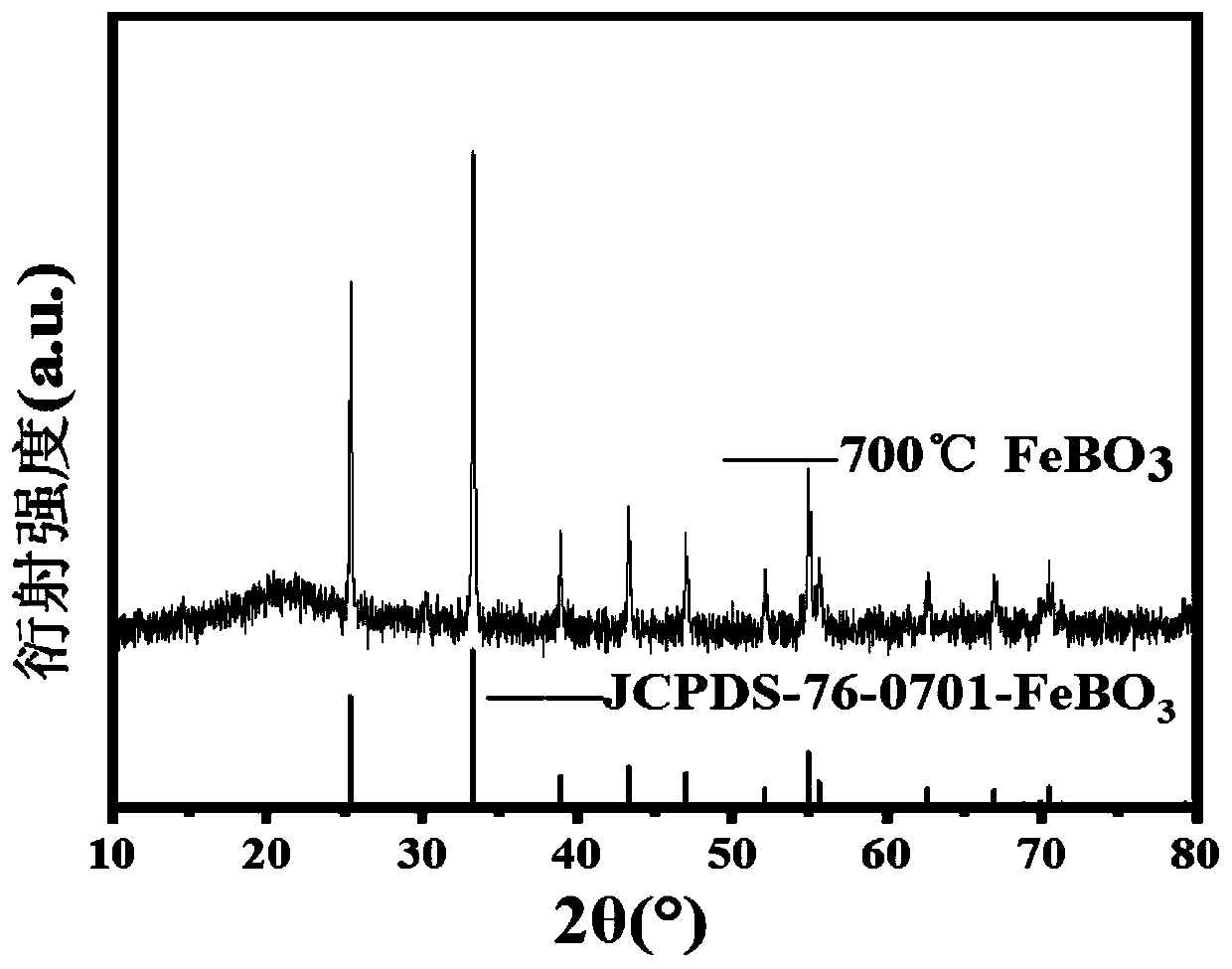
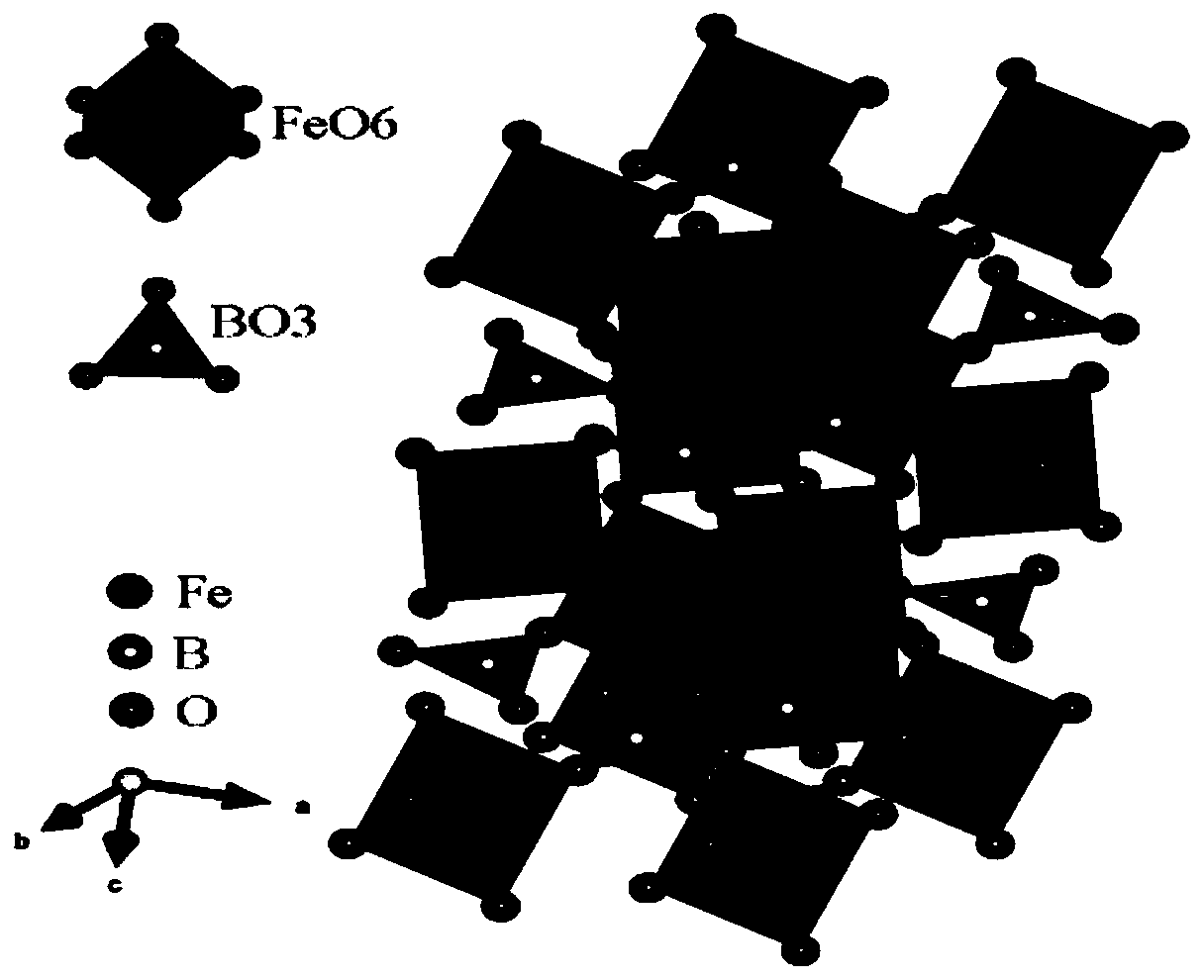
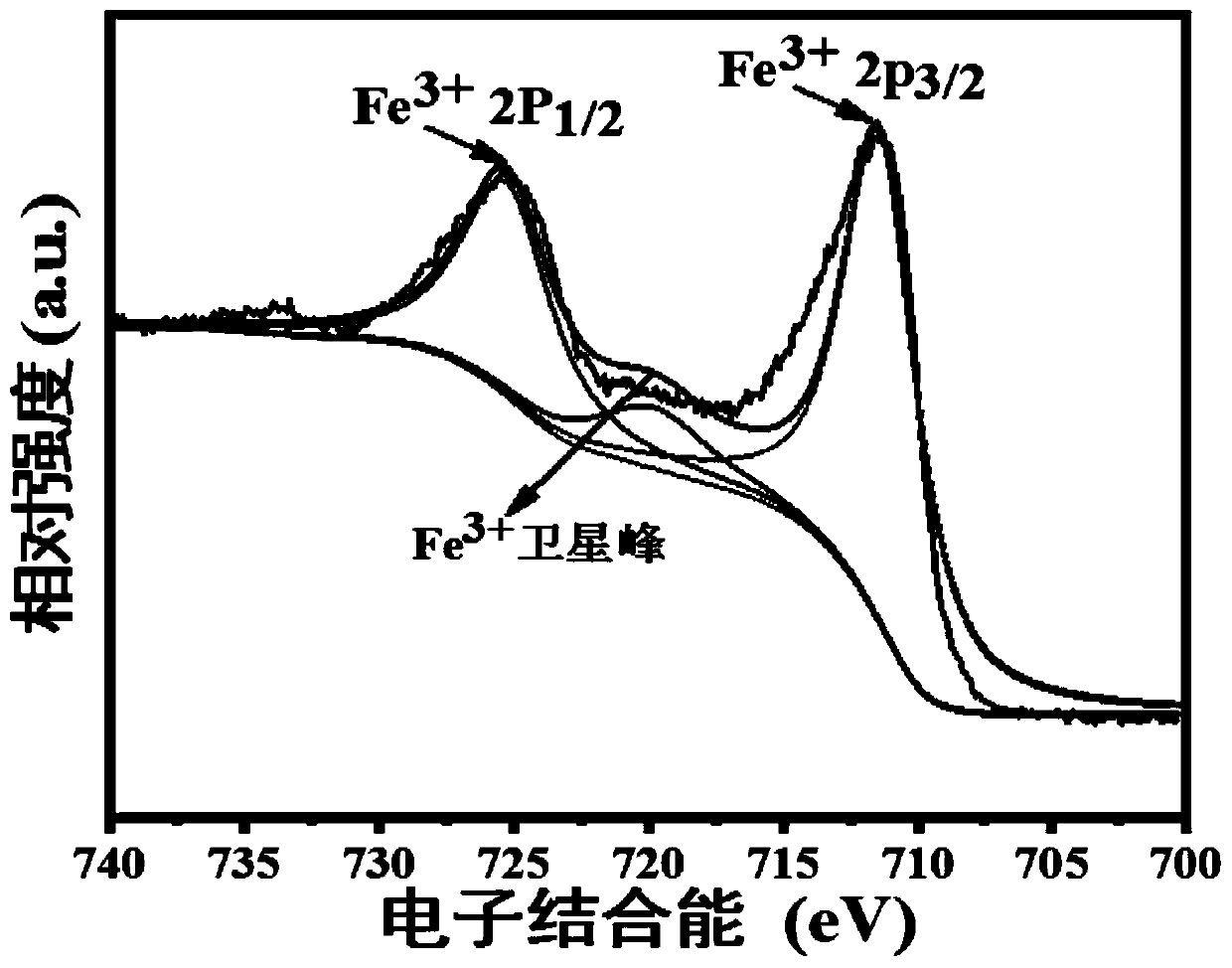
The invention discloses a novel negative electrode material of a sodium ion battery as well as preparation and application of the novel negative electrode material. A preparation process comprises steps: uniformly mixing an iron source and a boron source, and sintering to obtain the negative electrode material for the sodium ion battery, wherein the iron source comprises one or more of ferrous oxalate, ferrous acetate, ferrous citrate, ferric nitrate and ferric sulfate, and the boron source comprises one or more of boric acid, ammonium borate and diboron trioxide. The negative electrode material FeBO3 has the advantages of low raw material price, simple required equipment, less energy consumption for generating the FeBO3 material, short required time, high material purity and the like, isexcellent in electrochemical performance, and meets the requirements of high specific capacity, low cost and environmental protection of the negative electrode material of the sodium ion battery.
Owner:SHANGHAI UNIVERSITY OF ELECTRIC POWER
A kind of ash batch material with the function of catalytic degradation of formaldehyde and its preparation method
ActiveCN110975596BPromote absorptionOrganic-compounds/hydrides/coordination-complexes catalystsDispersed particle separationPolyvinyl alcoholPhosphate
Owner:南京冰锋环保科技有限公司
Nitrogen-phosphorus flame retardant and preparation method thereof
The invention relates to the technical field of flame retardants, and discloses a nitrogen-phosphorus flame retardant and a preparation method thereof. The nitrogen-phosphorus flame retardant comprises the following components in percentage by weight: 5%-10% of a nano flame retardant, 5%-8% of a nitrogen-phosphorus flame retardant, 5%-10% of a vulcanizing agent, 3%-5% of hydrotalcite, 5%-10% of montmorillonite, 10%-15% of zirconium phosphate, 1%-5% of graphite oxide, 5%-10% of a flame-retardant synergist, 1%-5% of boric acid, 2%-5% of zinc borate, 1%-5% of boron phosphate, 5%-9% of silicon dioxide, 1%-5% of aluminum hydroxide, 1% 4% of magnesium hydroxide, 2%-5% of phosphorus oxychloride, 3%-6% of molybdenum disicide and 4%-8% of pyrophosphoric acid. The nano flame retardant is crushed by the crusher, water is added into the nitrogen-phosphorus flame retardant for mixing, the hydrotalcite is heated, the silicon dioxide is crushed by the crusher, and the graphite oxide is ground by a grinding machine. The nitrogen-phosphorus flame retardant and the preparation method thereof have the advantages that the nitrogen-phosphorus flame retardant is convenient to stretch, the breaking strength of the flame retardant is high, the production cost is reduced, the process steps are simplified, the production efficiency is improved, and large-scale production is convenient.
Owner:上海聚千新材料发展有限公司
Process for preparing benzothiophen-2yl boronate
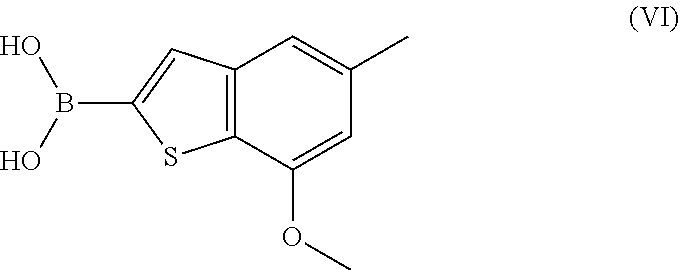
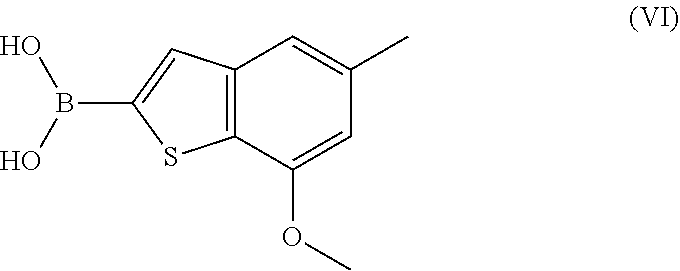
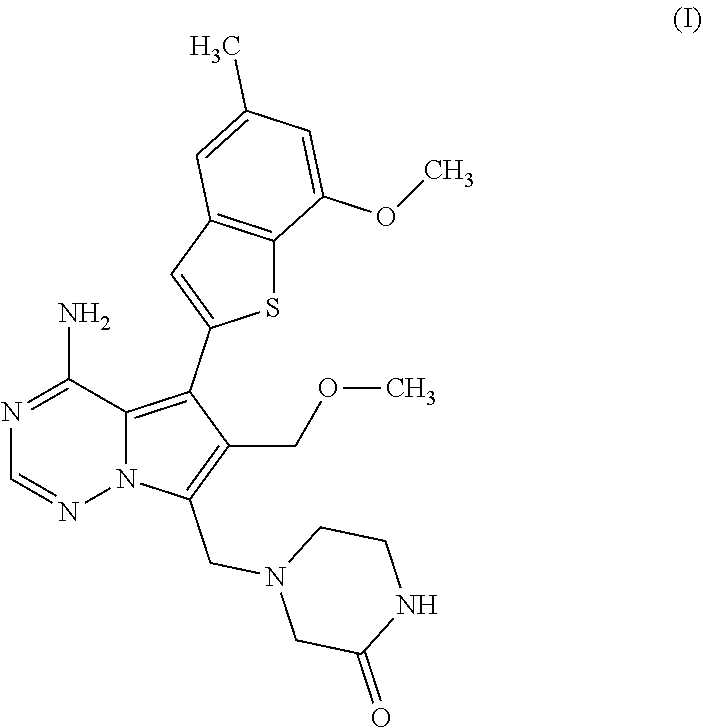
ActiveUS11274110B2Boron compound active ingredientsGroup 3/13 element organic compoundsDiseaseBoronic acid
A process for preparing the benzothiophen-2-yl boronate of formula (VI) which serves as an intermediate for production of medicaments and for production of medicaments for treatment and / or prophylaxis of proliferative disorders, such as cancer and tumor diseases.
Owner:BAYER PHARMA AG
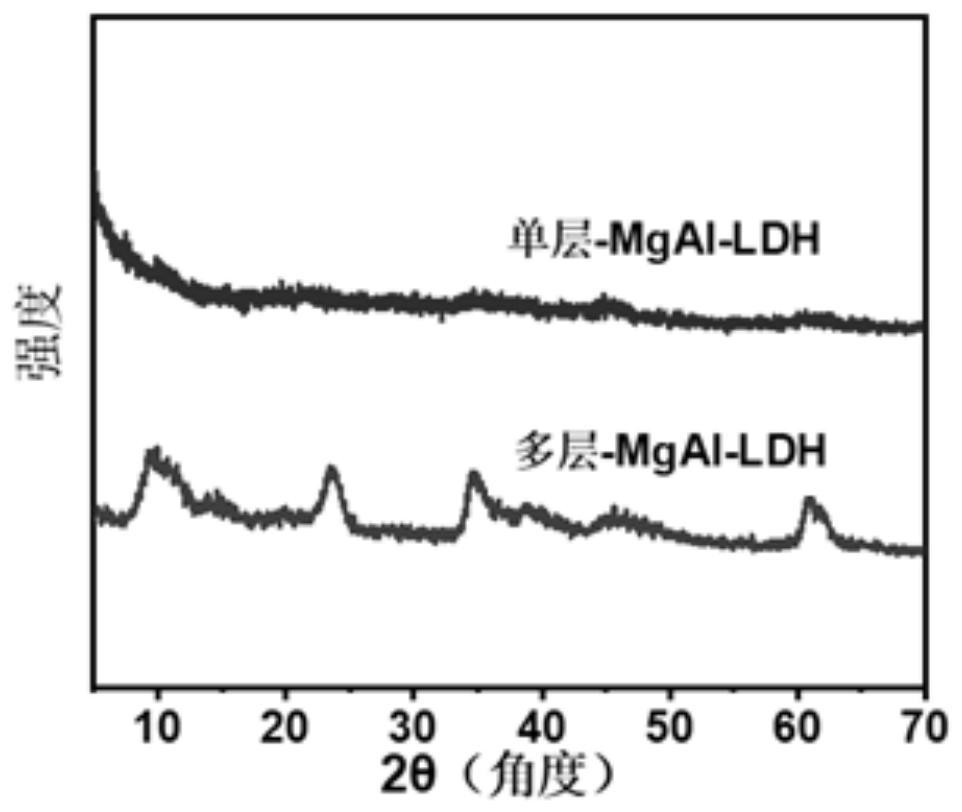
Single-layer hydrotalcite nano material and application thereof in efficient mineralization removal of high-concentration heavy metal ions in wastewater
PendingCN113877520AIncrease the adsorption rateIncrease capacityOther chemical processesWater contaminantsHigh concentrationMembrane reactor
The invention discloses a single-layer hydrotalcite nano material and an application thereof in efficient mineralization removal of high-concentration heavy metal ions in wastewater. Boric acid is added into a mixed solution of soluble divalent metal salt and soluble trivalent metal salt, the boric acid is combined in an aqueous solution to obtain [B4O5(OH)4]<2->, then the [B4O5(OH)4]<2-> and an alkali solution are simultaneously added into a full-backmixing rotating liquid membrane reactor to obtain intercalated hydrotalcite with uniform particle size, and the relatively large steric hindrance of the [B4O5(OH)4]<2-> increases the interlayer plate spacing of the hydrotalcite; and then peeling is carried out by using an interlayer plate inhibitor acetone, finally, washing is carried out to be neutral by using ethanol to obtain a single-layer hydrotalcite nanosheet, and the single-layer hydrotalcite nanosheet is dried to obtain single-layer hydrotalcite nano material powder. The prepared single-layer hydrotalcite nano material has a large specific surface area, can provide more adsorption active sites, improves the adsorption rate and capacity of heavy metal ions, and can achieve a high-speed and efficient removal effect on common heavy metal ions in various electroplating wastewater.
Owner:BEIJING UNIV OF CHEM TECH
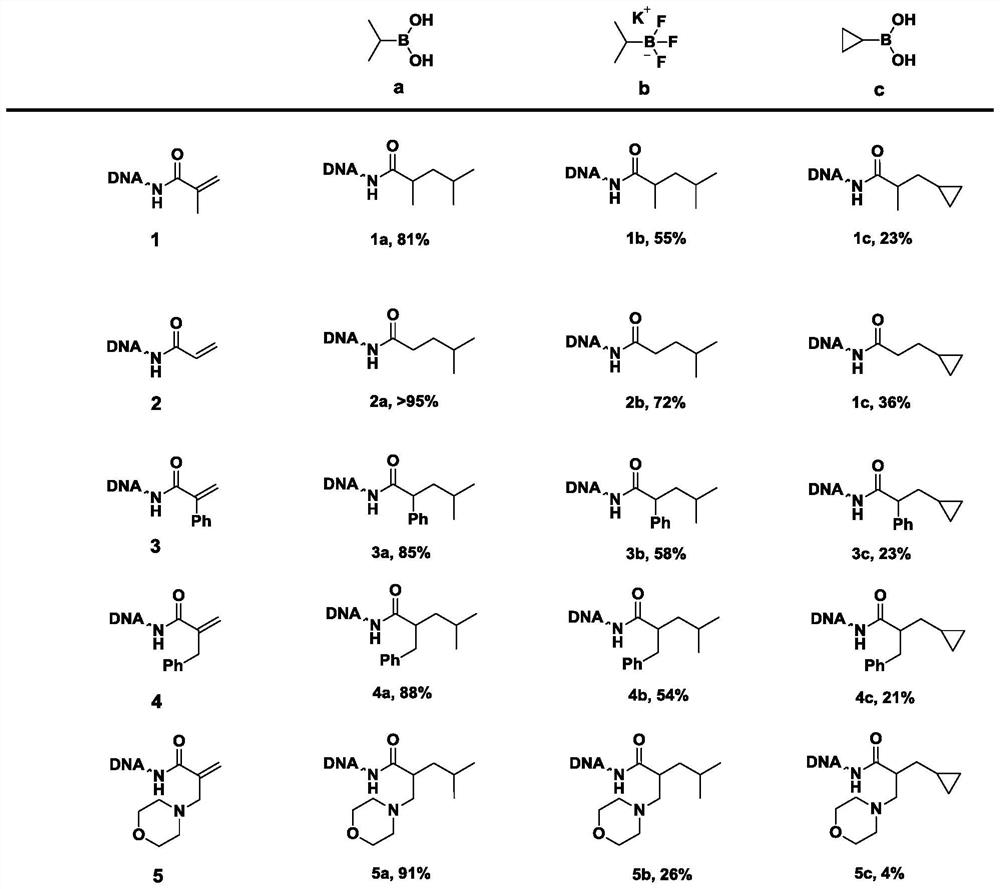
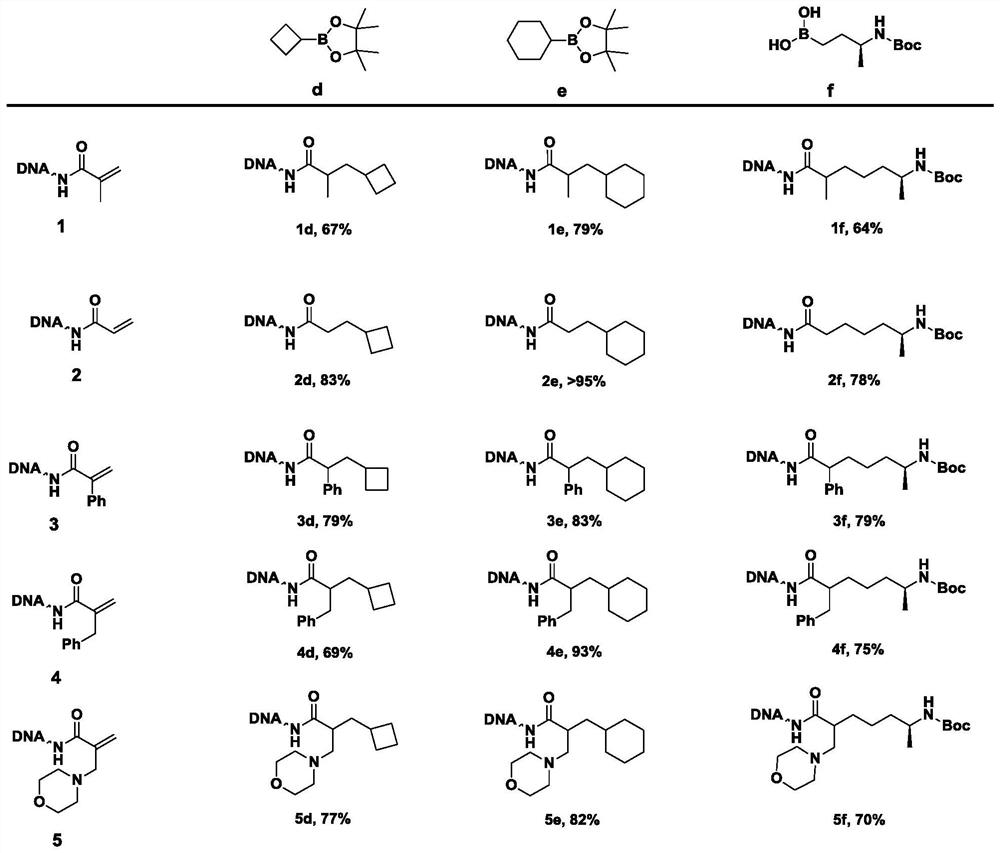
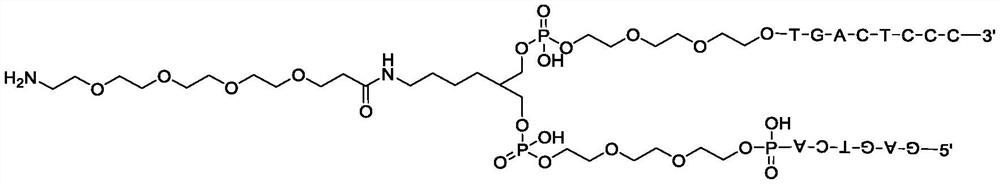
Method for constructing beta-fat substituted ketone compound through On-DNA reaction
The invention relates to a method for constructing a beta-fat substituted ketone compound through On-DNA (deoxyribonucleic acid) reaction, which is characterized in that an On-DNA alpha, beta-unsaturated carbonyl compound is used as a raw material and reacts with fat boric acid / boric acid ester or trifluoroborate in the presence of a photosensitizer and alkali to obtain the beta-fat substituted ketone compound. The method disclosed by the invention can be carried out in a mixed water phase of an organic solvent / water phase, the post-treatment is simple, the condition is mild, a high-diversity DNA coding compound library can be obtained in a relatively short time at a high yield, and the method is suitable for synthesizing DNA coding compounds by a porous plate.
Owner:HITGEN INC
Chemical forming solution, chemical forming method and anode foil
ActiveCN111139508BImprove bindingImprove specific volume reductionAnodisationElectrolytic capacitorsAmmonium borateCapacitor
The present invention provides a laminated foil chemical conversion solution and chemical conversion method, and an anode foil obtained by the chemical conversion method. The chemical forming method includes: pretreatment; boiling; four-stage chemical forming in a strong reducing agent chemical forming solution; first heat treatment; first immersion; first supplementary forming treatment; second immersion; ; The second heat treatment; the third complementary forming treatment; the third dipping; The strong reducing agent chemical conversion solution is an aqueous solution containing 2-10wt% boric acid, 0.01-1wt% ammonium borate and 0.001-10wt% strong reducing agent, and the anode foil obtained by chemical conversion using the chemical conversion liquid has a high specific volume and is suitable for use in Electrode material for aluminum electrolytic capacitors.
Owner:DONGGUAN DONGYANG SOLAR SCI RES & DEV CO LTD
Suzuki reaction method of aryl boric acid/boric acid ester containing large steric hindrance substituent
PendingCN113831319AMild reaction conditionsReduce experiment costOrganic chemistryPtru catalystPalladium catalyst
The invention provides a Suzuki reaction method of aryl boric acid / boric acid ester containing a large steric hindrance substituent, the reaction has the characteristics as shown in a formula I, and Ar represents a compound with a conjugated structure; R1 represents alkyl containing 2-18 carbon atoms, Y1 represents alkyl containing 1-18 carbon atoms, alkoxy, alkylthio, ester group and other groups; and B represents a di-oxapentaborane group or a boric acid group. According to the method provided by the invention, proper solvents such as ethylene glycol dimethyl ether, diethylene glycol dimethyl ether and the like are matched with a common palladium catalyst to form a reaction system, so that the problem that Suzuki reaction with large steric hindrance groups is difficult to carry out is effectively solved.
Owner:UNIV OF SCI & TECH BEIJING +1
Preparation method of Ir-O-P type catalyst diboric acid/ester compound
ActiveCN113980044AHigh yieldStrong designabilityIndium organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsArylPtru catalyst
The invention discloses a preparation method of an Ir-O-P type catalyst diboronic acid / ester compound, and belongs to the technical field of organic synthesis. The preparation method comprises the following steps that under the air condition, using an Ir metal complex and a phosphine ligand to form an active Ir-O-P type catalyst; and adding an organic boron source [B], a beta-N-aryl (alkyl) substrate and the Ir-O-P type catalyst into an organic solvent under anhydrous and anaerobic conditions, conducting reacting at 60-120 DEG C, and after the reaction is finished, performing post-treatment to obtain the aryl (alkyl) diboronic acid / borate compound. According to the reaction, the aryl (alkyl) diboronic acid / borate compound can be obtained through a one-pot method, and the limitation that the compound needs to be synthesized in multiple steps and the substrate range is limited is made up. In addition, in the preparation of the aryl triazene diboride, even if no ligand is added, a target compound can be obtained at a high yield, and based on the advantages, the method shows a very high yield after being expanded to a gram-level reaction, and lays a good foundation for application in the field of drug synthesis or material development.
Owner:XI AN JIAOTONG UNIV
Material with low humidity sensitivity and high saturation water absorption and preparation method thereof
ActiveCN114316276AHigh saturated water absorptionImprove water absorptionOther chemical processesBoronic acidMoisture absorption
The invention provides a material with low humidity sensitivity and high saturation water absorption capacity and a preparation method thereof, and relates to the technical field of water absorption material preparation. Hydroxyl siloxane and a boron-containing compound mixture react to prepare the low-humidity sensitive high-water-absorption material, and the hydroxyl siloxane is selected from any one of a hydroxyalkyl-containing compound, a silanol structure-containing compound and a hydroxyalkyl-containing and silanol-containing polymer, and the boron-containing compound is selected from a boron-containing compound, a boron-containing compound, a boron-containing compound, a boron-containing compound, a boron-containing compound, a boron-containing compound and a boron-containing compound. The boron-containing compound is selected from any one or more of boric acid, boric anhydride and boric acid ester; the prepared water absorption material has good moisture absorption activity in a low-humidity environment, the saturated water absorption capacity is larger than 55 wt% in an environment with the relative humidity being 4%, meanwhile, the dehydration temperature of a saturated material adsorbing water is larger than 80 DEG C in dry nitrogen, and the water absorption material has high thermal stability.
Owner:GENERAL ENG RES INST CHINA ACAD OF ENG PHYSICS
Steel surface coating and preparation method thereof
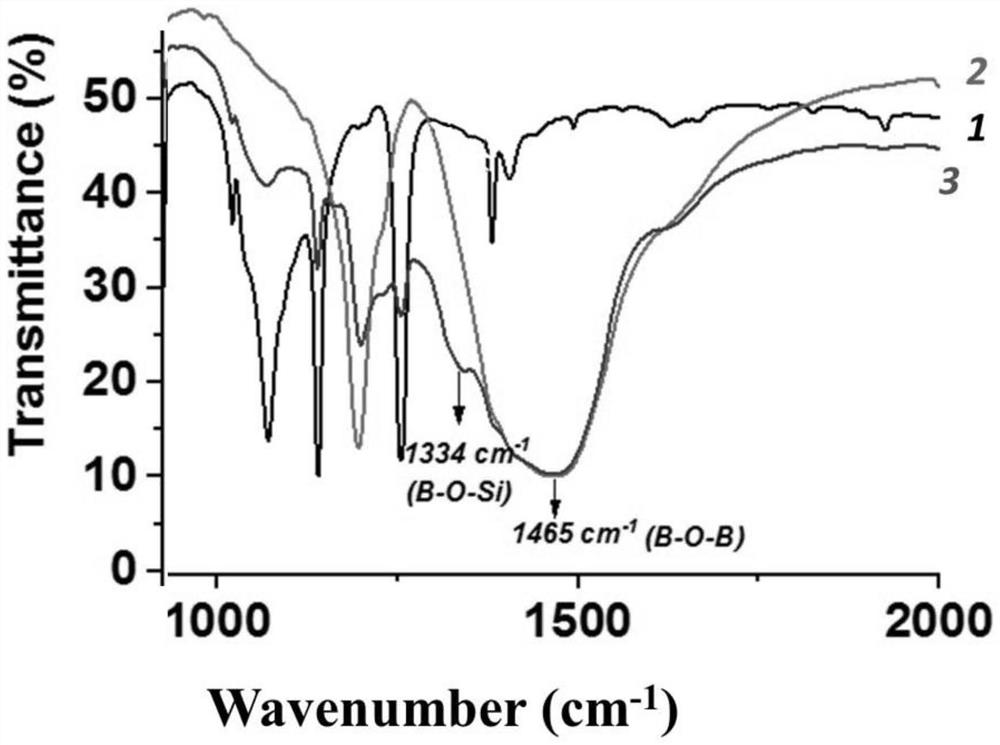
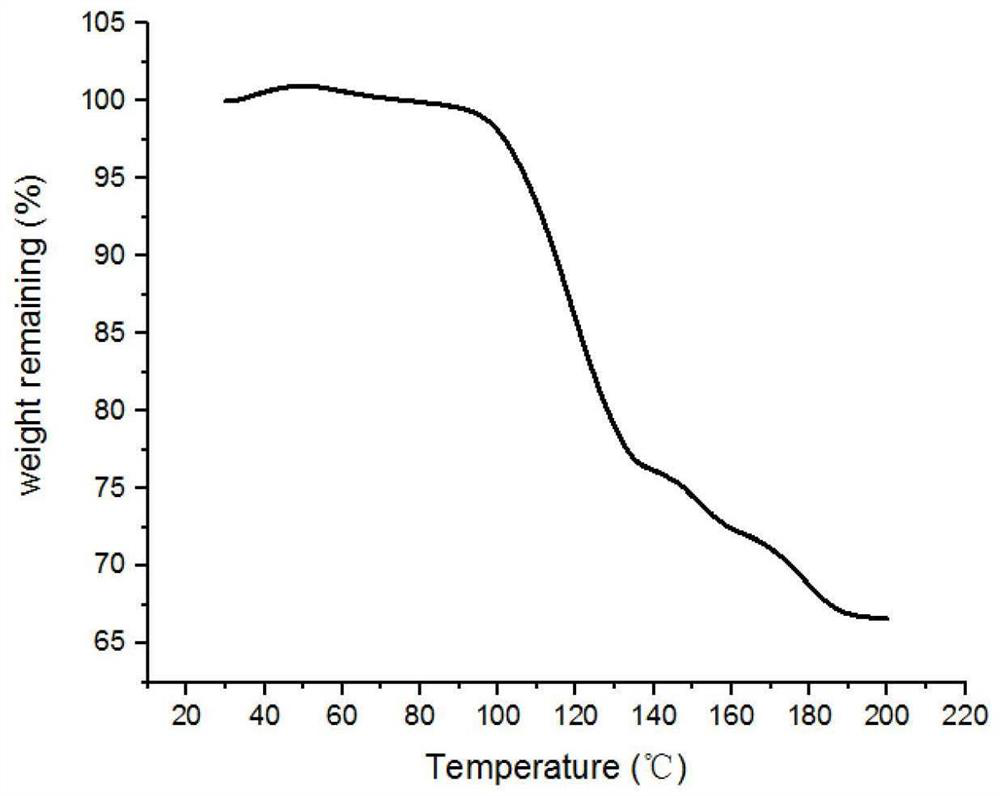
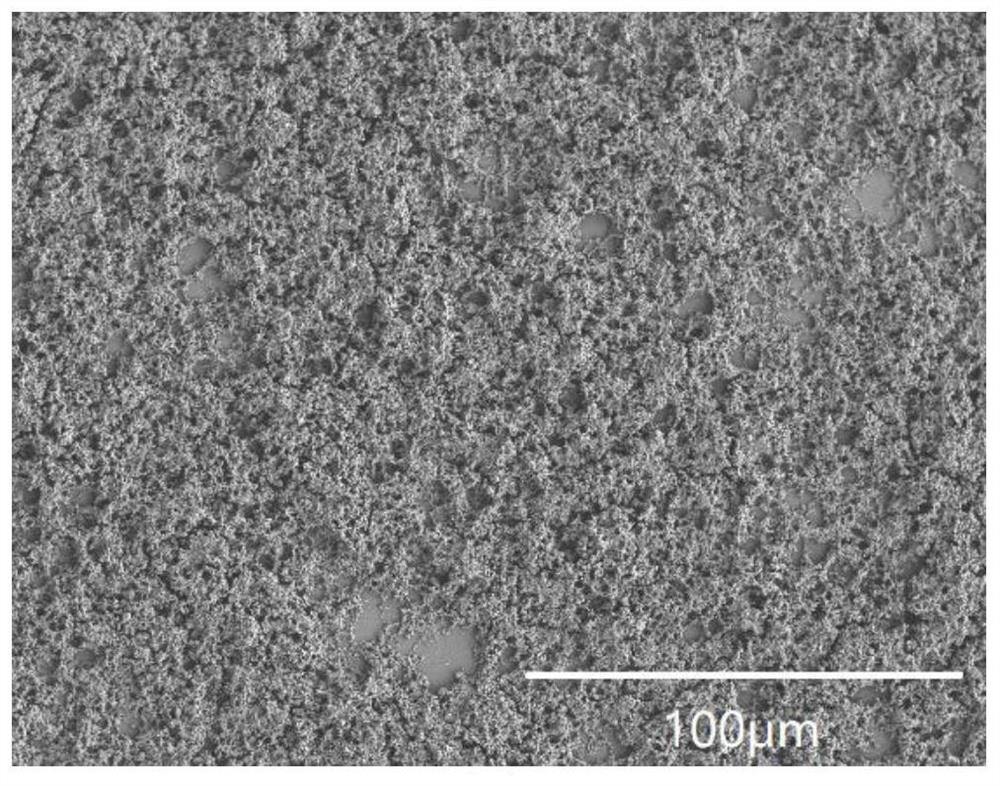
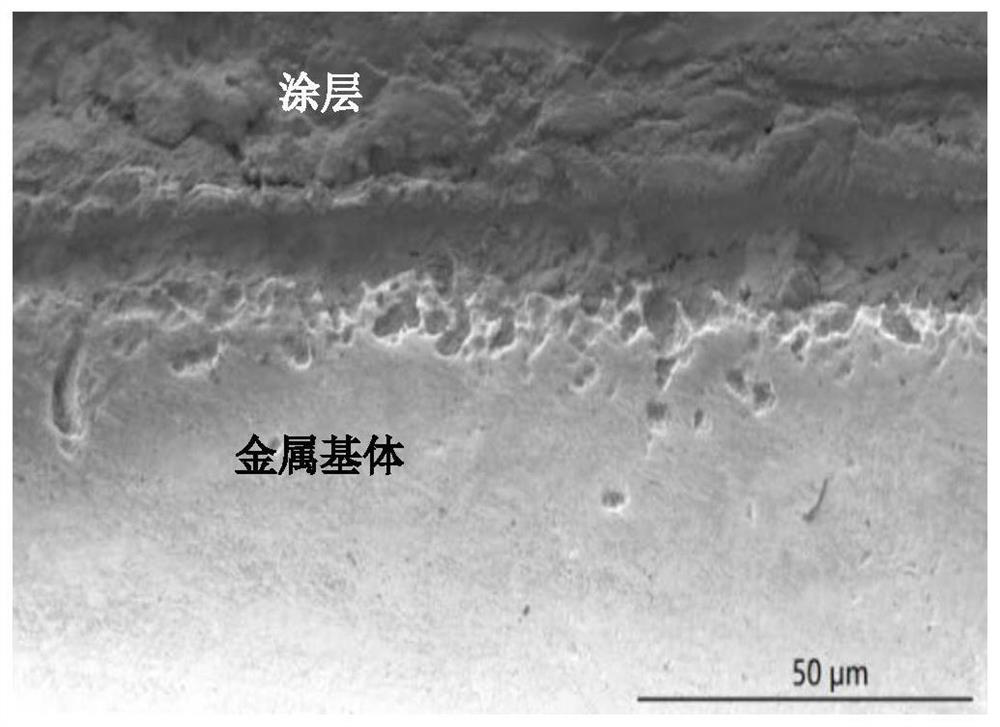
InactiveCN112898900AEasy to prepareImprove bindingAnti-corrosive paintsProtein coatingsBinding forceBorate Boric Acid
The invention belongs to the technical field of preparation of biological composite materials, and particularly relates to a coating with certain protectiveness on the surface of steel and a preparation method of the coating. A coating solution is a mixed solution of a boric acid-sodium borate buffer solution and a mussel mucin stock solution. The preparation method is simple, the binding property of the coating and a steel substrate is good, and the coating has a good protection effect on the steel substrate. In order to keep stable performance of the mussel mucin stock solution, the mussel mucin stock solution is usually stored in an acidic environment, and a boric acid-sodium borate buffer solution is used for adjusting the environment of the mussel mucin stock solution from acidity to alkalinity; the mussel mucin can be cured on the surface of the steel substrate and form a biological coating layer with certain binding force and protectiveness under the condition of a proper ratio of a buffer solution to a mussel mucin stock solution, so that the service life of the steel in a water environment is prolonged.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com