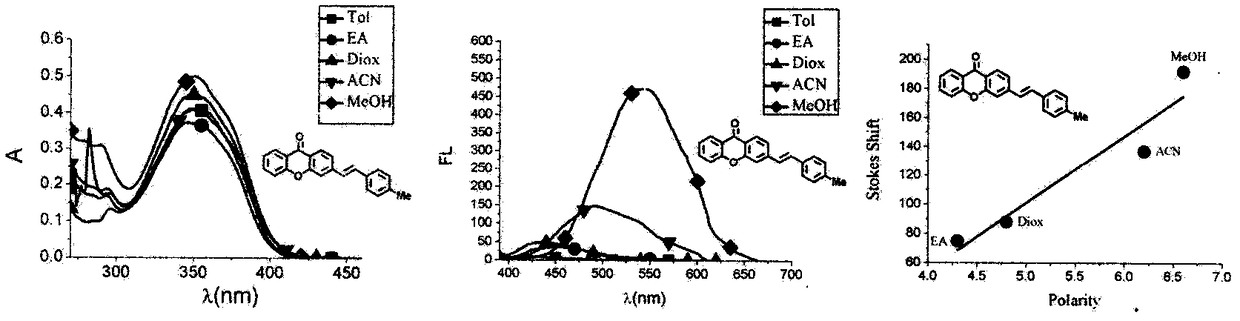
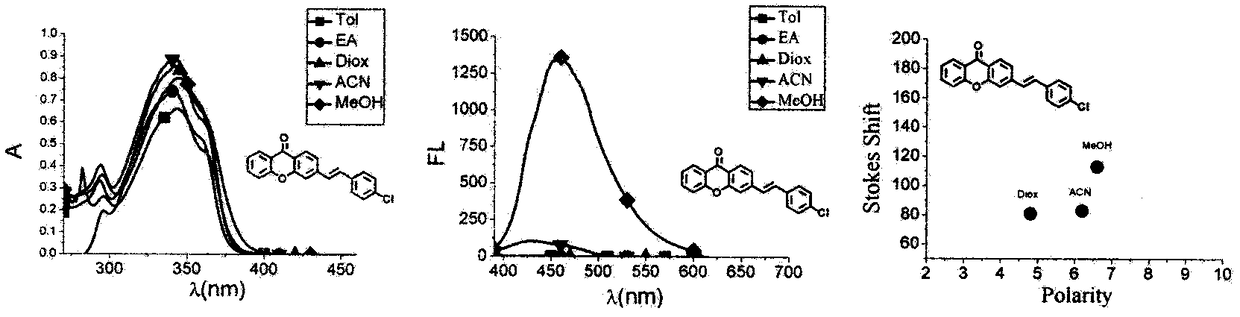
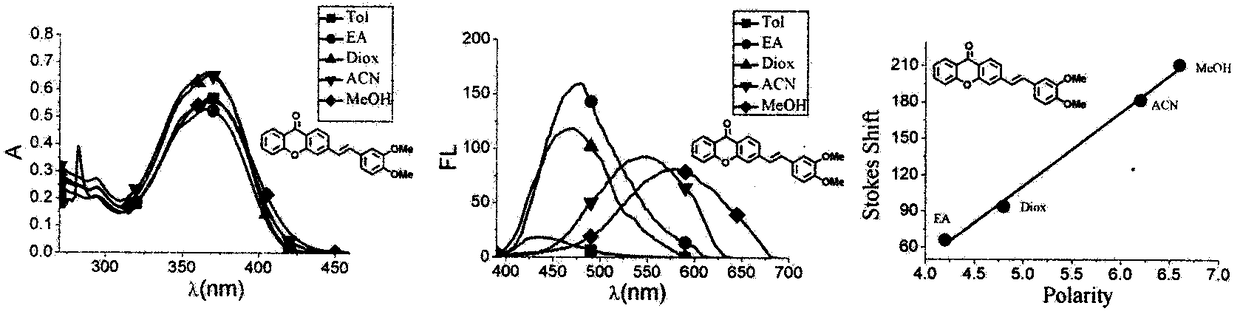
Xanthone fluorochrome and application
A technology of xanthone and heterocyclic aryl, which is applied in the direction of luminescent materials, organic dyes, fluorescence/phosphorescence, etc., can solve the problems of poor tissue permeability and short emission wavelength, and achieve the effect of simple synthesis and easy-to-obtain products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051]
[0052] (E)-3-(4-(dimethylamino)styryl)-9H-xanthen-9-one
[0053] Preparation of Compound (E)-3-(4-(dimethylamino)styryl)-9H-xanthen-9-one
[0054]
[0055] o-Nitrobenzaldehyde (20.0g, 0.13mol), m-cresol (18.6g, 0.17mol), anhydrous copper chloride (890mg, 6.6mmol), triphenylphosphine (2.6g, 10mmol) and anhydrous Potassium phosphate (62.0g, 0.29mol) was dissolved in 100 ml of redistilled toluene, and the reaction solution was refluxed at 110°C; after 24 hours of reaction, the reaction solution was cooled to room temperature, filtered with diatomaceous earth, and washed with ethyl acetate several times. The filter cake was cleaned; then the solvent was evaporated to dryness by distillation under reduced pressure. After dissolving the residue with 150 ml of ethyl acetate, add an equal amount of water and wash it several times until the color of the water phase becomes clear from dark brown; then wash it several times with 40% aqueous sodium hydroxide solution until...
Embodiment 2
[0059]
[0060] (E)-3-(4-hydroxystyryl)-9H-xanthen-9-one
[0061] Preparation of Compound (E)-3-(4-hydroxystyryl)-9H-xanthen-9-one
[0062]
[0063] According to Example 1, compound c was prepared. Compound c (224mg, 0.1mmol), p-hydroxybenzyltriphenylphosphonium salt (434mg, 0.1mmol), sodium ethoxide (20.4mg, 0.3mmol) were added to a 50ml round bottom reaction flask, dissolved in 10ml of DMF, at room temperature Under stirring reaction for 24 hours, the reaction solution changed from initial dark rose red to light yellow, adding 10 milliliters of water to quench the reaction, extracting with 20 milliliters of ethyl acetate, separating the aqueous phase, and washing with 10 milliliters of water 5 to 8 times, fully DMF was removed, and finally dried over anhydrous sodium sulfate; the organic phase was distilled under reduced pressure, purified by column chromatography, and loaded by dry method (PE: EA = 20: 1) to obtain 240 mg of the target compound in the form of light y...
Embodiment 3
[0065]
[0066] (E)-3-(4-nitrostyryl)-9H-xanthen-9-one
[0067] Preparation of Compound (E)-3-(4-nitrostyryl)-9H-xanthen-9-one
[0068] According to Example 1, compound c was prepared. Compound c (224mg, 0.1mmol), p-nitrobenzyltriphenylphosphonium salt (434mg, 0.1mmol), sodium ethoxide (20.4mg, 0.3mmol) were added to a 50ml round bottom reaction flask, dissolved in 10ml of DMF, Stirring and reacting at room temperature for 24 hours, the reaction solution changed from initial dark rose red to light yellow, adding 10 milliliters of water to quench the reaction, extracting with 20 milliliters of ethyl acetate, separating the aqueous phase, and washing with 10 milliliters of water for 5 to 8 times. DMF was fully removed, and finally dried over anhydrous sodium sulfate; the organic phase was distilled under reduced pressure, purified by column chromatography, and loaded by dry method (PE: EA = 20: 1) to obtain 240 mg of the target compound as a light yellow powder, with a yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com