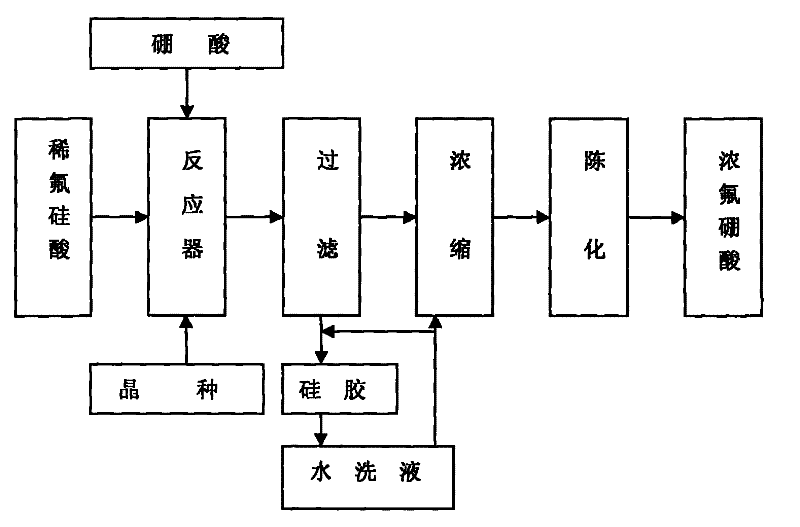
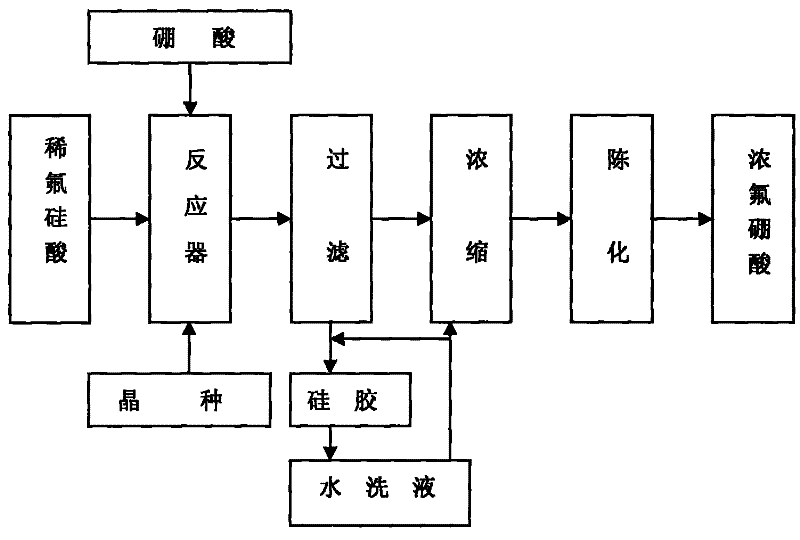
Method for preparing fluoroboric acid using by-product fluosilicic acid with low concentration in wet method phosphoric acid production process
A wet-process phosphoric acid and fluosilicic acid technology, which is used in the removal of boron halide compounds and solid waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 480.0g of 6.67% fluorosilicic acid solution, put it in the reactor, add 3.3374g of silicon dioxide, start stirring to fully disperse the silicon dioxide in the fluorosilicic acid solution, preheat to 54°C, and adjust the stirring speed to 56r / min, weigh 20.67g of 99.7% boric acid (n=1.0), slowly add it into the reactor, the feeding time is 60min, the temperature of the slurry is raised to 80°C, constant temperature, after 2h of reaction, suction filtration under the condition of -0.078MPa , Obtain 345.6 g of filtrate. Concentrate at 75°C and -0.07MPa to obtain 38.70g of a 40.21% concentrate. Wash the filter cake three times with 250ml, and combine to obtain 274.2g of washing liquid. The technical indicators of the filtrate after concentration are shown in Table 1.
[0024] Table 1
[0025] project index Exterior colorless transparent liquid Fluoboric acid (HBF 4 ) mass score 40.21% Free boric acid (H 3 BO 3 ) quality score ...
Embodiment 2
[0027] Weigh 250g of 9.28% fluosilicic acid solution, put it in the reactor, add 1.9278g of silicon dioxide, start stirring to fully disperse the silicon dioxide in the fluosilicic acid solution, preheat to 50°C, and adjust the stirring speed to 60r / min, weighed 15.72g of 99.7% boric acid (n=1.05), slowly added to the reactor, the feeding time was 65min, the temperature of the slurry was raised to 85°C, kept at constant temperature, and reacted for 2.5h, and then pumped under the condition of -0.050MPa Filtrate to obtain 192.5 g of the filtrate, which was concentrated at 70° C. and -0.065 MPa to obtain 27.35 g of a 40.06% concentrated solution. Wash the filter cake with 274.2 g of the washing solution obtained in Example 1 to obtain 264.2 g of the secondary washing solution, then rinse the filter cake twice with 100 ml of distilled water, and combine to obtain 89.3 g of the washing solution. The technical indicators of the filtrate after concentration are shown in Table 2.
...
Embodiment 3
[0032] Weigh 250g of 10.82% fluosilicic acid solution, put it in the reactor, add 1.6917g of silicon dioxide, start stirring to fully disperse the silicon dioxide in the fluosilicic acid solution, preheat to 53°C, and adjust the stirring speed to 50r / Min, weigh 16.59g of 99.7% boric acid (n=0.95), slowly add it into the reactor, the feeding time is 70min, the temperature of the slurry is raised to 90°C, keep the temperature constant, after reacting for 3h, suction filter under the condition of -0.050MPa , to obtain 224.2 g of filtrate, which was concentrated under the conditions of 80° C. and -0.06 MPa to obtain 34.62 g of 41.02% concentrated solution. Rinse the filter cake with the secondary washing of Example 1 and Example 2 to obtain 261.3 g of washing liquid three times, then rinse the filter cake twice with 100 ml of distilled water, combine to obtain washing liquid 103.7 g, and merge with the first washing liquid of Example 2 . The technical indicators of the filtrate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com