Preparation method of Ir-O-P type catalyst diboric acid/ester compound
An ester compound and catalyst technology, applied in the field of organic synthesis, achieves the effects of strong designability, good compatibility and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A kind of preparation method of Ir-O-P type catalyst diboronic acid / ester compound comprises the steps: under anhydrous and oxygen-free condition, organic boron source [B], β-N-aryl (alkane) substrate, metal iridium catalyst and The organic phosphine ligand is added into the organic solvent and reacted at 60-120° C. After the reaction is completed, the aryl (alkyl) diboronic acid / boric acid ester compound is obtained through post-treatment.
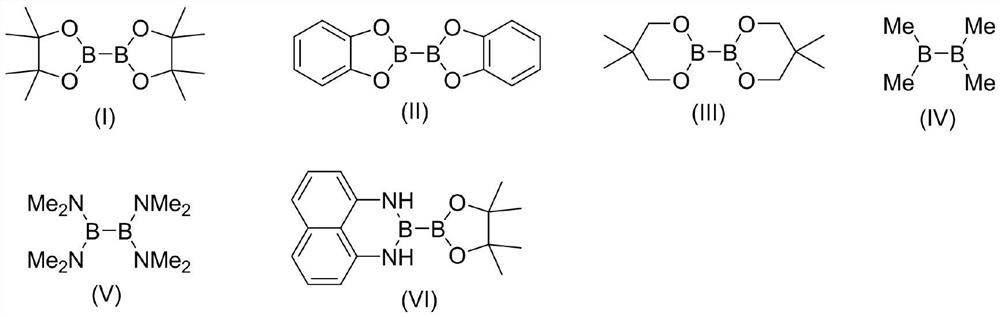
[0046] The structure of the organic boron source [B] is shown in formulas (I) to (IV):
[0047]
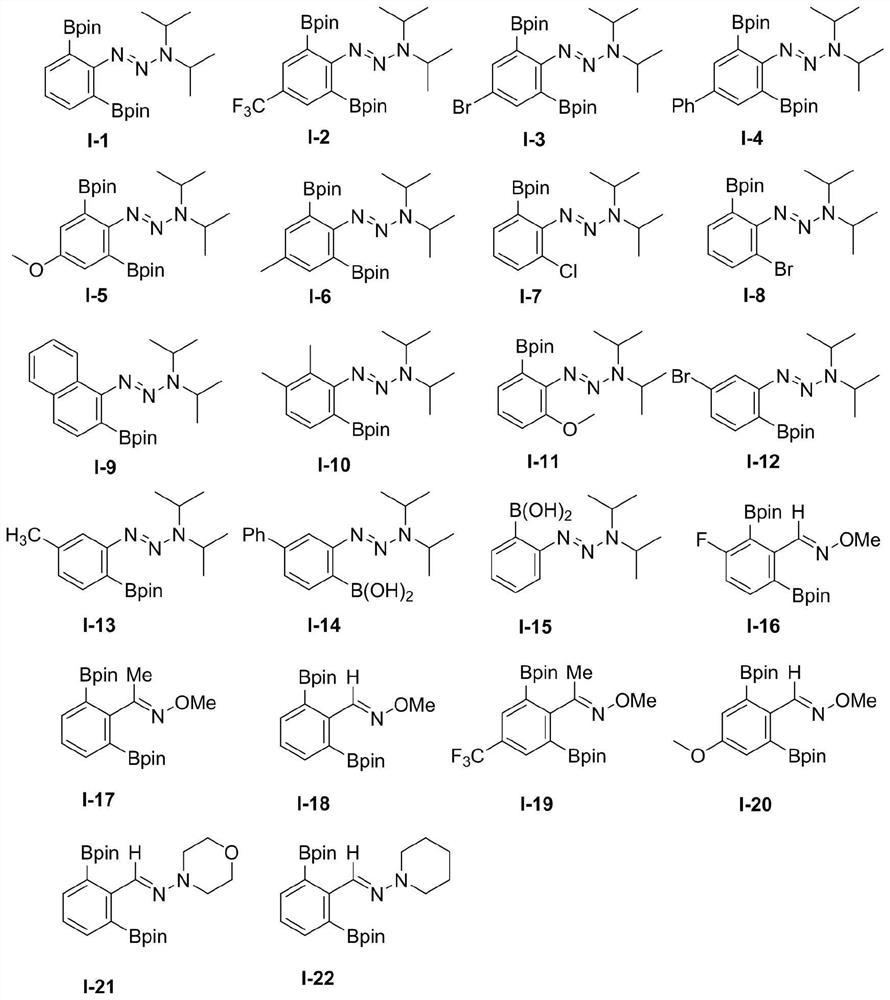
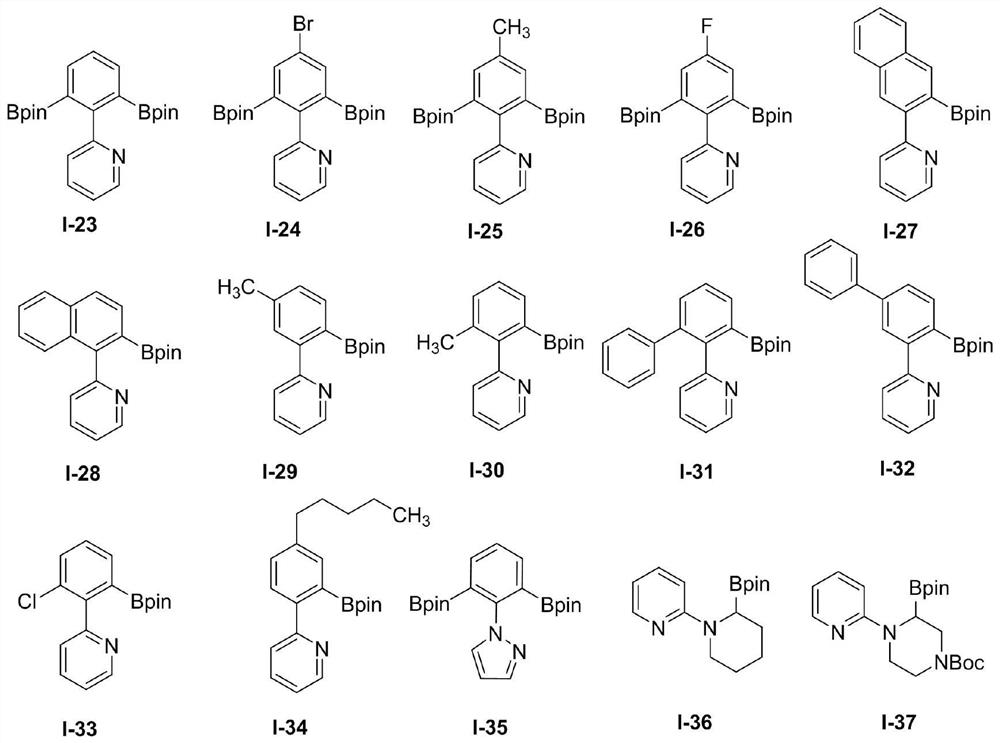
[0048] The structure of the β-N-aryl (alkane) base substrate is shown in formulas (IV) to (VII):
[0049]
[0050] The structure of described aryl (alkyl) base diboronic acid / boric acid ester compound is as shown in the formula:
[0051]
[0052] The molar ratio of the β-N-aryl(alkane)-based substrate to the organic boron source [B] is 1.0:1.0-4.0.
[0053] The reaction formula is as follows:
[0054]
[0055] After our st...
Embodiment 2
[0068] Add methoxycyclooctadiene iridium, triphenylphosphine, β-N-aryl (alkane) base substrate, bispinacol borate, organic solvent 2mL in the Schlenk tube of 10mL according to the raw material ratio of Table 1, React with heating and stirring at 60-120°C. After the reaction is completed according to the reaction conditions in Table 2, concentrate, mix the sample with silica gel, and purify by column chromatography to obtain the corresponding aryl (alkyl) diboronic acid / boric acid ester compound. The reaction process is as follows: Shown:
[0069]
[0070] Table 1
[0071]
[0072]
[0073]
[0074] The reaction conditions of the foregoing examples of table 2 and the resulting product
[0075]
[0076]
[0077]
[0078] In Table 2, T is the reaction temperature, and t is the reaction time.
[0079] Structural confirmation data of some compounds
[0080] The nuclear magnetic resonance (NMR) ( 1 H NMR and 13 C NMR) detection data are:
[0081]
[008...
Embodiment 3
[0096] The invention also discloses a water-resistant and oxygen-resistant Ir-O-P bond-containing novel metal iridium catalyst when preparing aryl (alkyl)-based diboronic acid / boric acid ester compounds, which can be widely used in various C-H bond boronations, substrates Good tolerance, catalytic activity is better than all kinds of commercially available metal iridium catalysts.
[0097] The beneficial effects of the present invention are:
[0098] 1. The preparation method of the Ir-O-P type catalyst diboronic acid / ester compound obtains the aryl (alkyl) base diboronic acid / boric acid ester compound through a one-pot method, which makes up for the need for multi-step synthesis of this type of compound and the limited range of substrates However, in the preparation of aryl triazene diborides, the target compound can be obtained in a higher yield even without adding a ligand. The high yield lays a good foundation for the application in the field of drug synthesis or material...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com