Suzuki reaction method of aryl boric acid/boric acid ester containing large steric hindrance substituent
A technology based on aryl boronic acid and Suzuki reaction, which is applied in the direction of organic chemistry, can solve the problems of difficult reaction of large hindered groups, high difficulty of synthesis, and industrialization of new catalysts, so as to reduce experimental costs, shorten reaction time, and reduce side effects. Response reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Preparation of 3,3'-bis(s-trimethylphenyl)-2,2'-bithiophene
[0029]
[0030] The specific description of the preparation reaction formula is as follows:
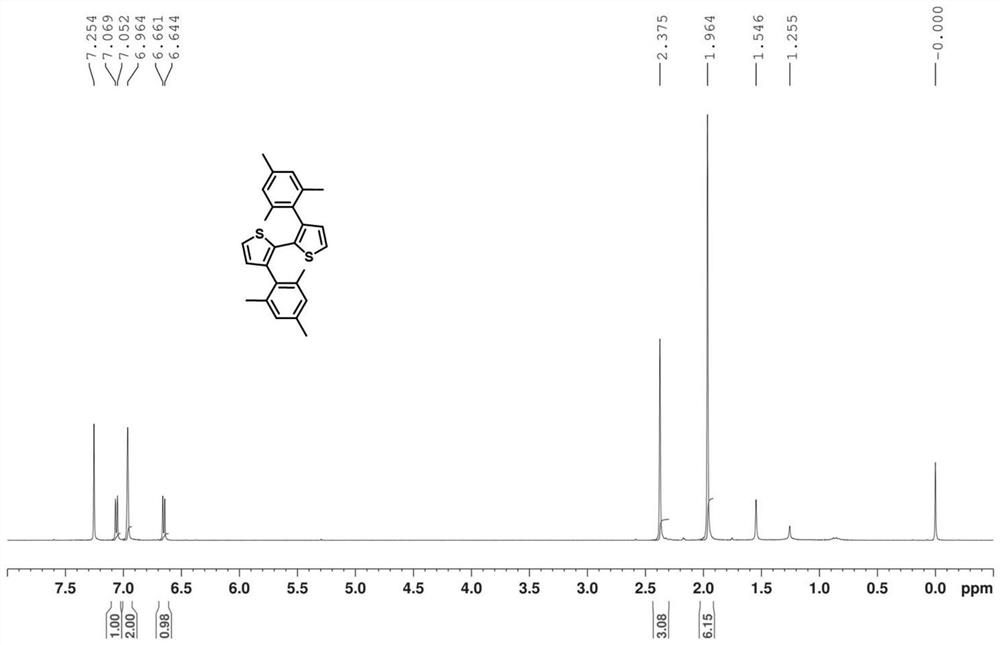
[0031] Add 2g of 3,3'-dibromo-[2,2'-bithiophene]-5,5'-bis(trimethylsilane) and 0.3g of tetrakis(triphenylphosphine)palladium into the two-necked flask, Stir and add 45ml of ethylene glycol dimethyl ether under an argon-protected atmosphere until dissolved, dissolve 2.8g of 2,4,6-trimethylphenylboronic acid in 30ml of ethylene glycol dimethyl ether and add to the reaction system, then add 3.3g Potassium tert-butoxide was dissolved in 30ml tert-butanol and added to the reaction system, reacted at 90°C for 0.5h, and purified by column chromatography to obtain a white solid 3,3'-di(mesityl)-2,2' -Bithiophene, yield 80%.
[0032] Such as figure 1 Shown, NMR analysis results: 1 H NMR (300MHz, CDCl 3 )(ppm): δ7.34(d,2H),7.16–7.10(m,6H),7.08(d,2H),7.06–7.00(m,4H).
[0033] Its molecular weight was 402.61 ...
Embodiment 2
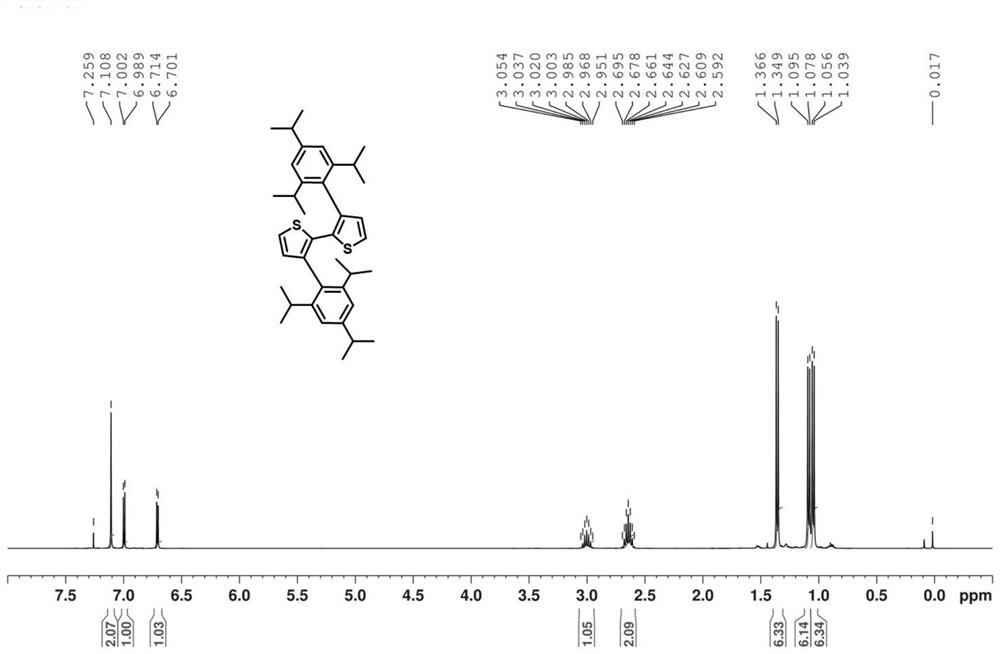
[0034] Example 2: Preparation of 3,3'-bis(2,4,6-triisopropylphenyl)-2,2'-bithiophene
[0035]
[0036] The specific description of the preparation reaction formula is as follows:
[0037] 1) when adopting the reaction condition identical with embodiment 1
[0038] Dissolve 2g of 3,3'-dibromobithiophene, 6.12g of (2,4,6-triisopropylphenyl)boronic acid, and 400mg of tetrakis(triphenylphosphine)palladium in ethylene glycol dimethyl ether, Finally, 4.85g of potassium tert-butoxide was dissolved in tert-amyl alcohol and added to the reaction system. The reaction was stirred in an oil bath at 90°C. The stirring was continued for about 96 hours. After purification by column chromatography, 3,3'-bis(2, 4,6-triisopropylphenyl)-2,2'-bithiophene is a white solid with a yield of 35%.
[0039] 2) when adopting another solvent system and temperature of reaction in this technical scheme
[0040] Since the steric hindrance effect in 2,4,6-triisopropylphenylboronic acid is relatively lar...
Embodiment 3
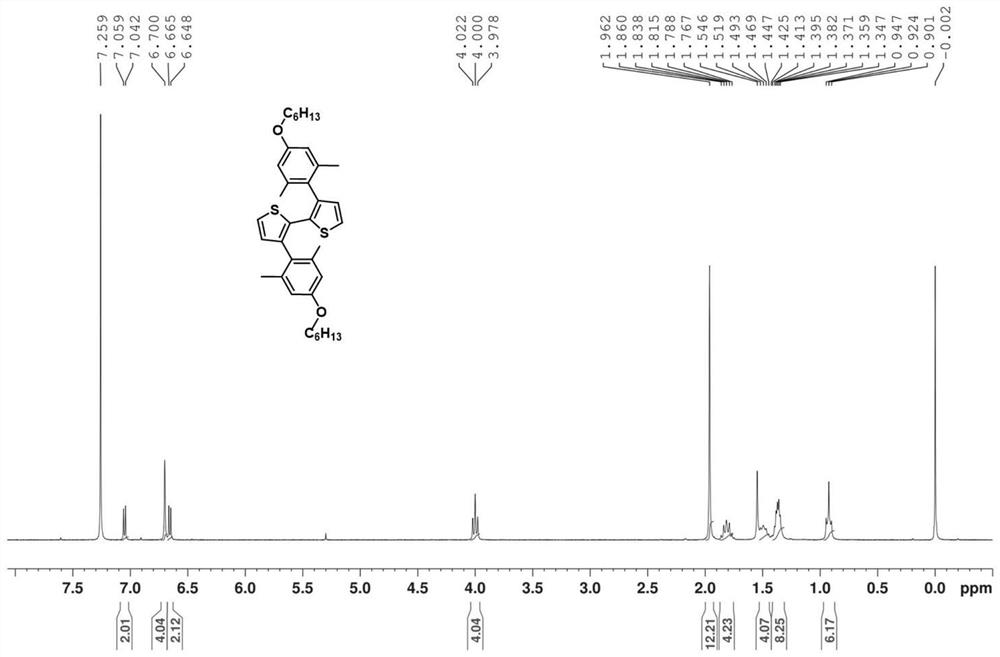
[0044] Example 3: Preparation of 3,3'-bis(4-hexyloxy-2,6-dimethylbenzene)-2,2'-bithiophene
[0045]
[0046] 1) when adopting the reaction condition identical with embodiment 1
[0047] 3.24g 3,3'-dibromobithiophene, 13.3g 2-(4-hexyloxy-2,6-xylene)-4,4,5,5-tetramethyl-1,3,2-di Dissolve oxaborane and 650mg tetrakis(triphenylphosphine)palladium in ethylene glycol dimethyl ether, then dissolve 7.86g potassium tert-butoxide in tert-amyl alcohol and add to the reaction system, and stir in an oil bath at 90°C reaction, the raw materials decompose, and the product cannot be obtained.
[0048] 2) When adopting the second reaction condition in Example 2, due to the further increase of temperature, the decomposition of raw materials is more rapid, and the product cannot be obtained.
[0049] 3) when adopting another solvent system, alkali and temperature of reaction in this technical scheme
[0050] 3,3'-dibromodithiophene 0.01mol, 2-((4-hexyloxy)-2,6-xylene)-4,4,5,5-tetramethyl-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com