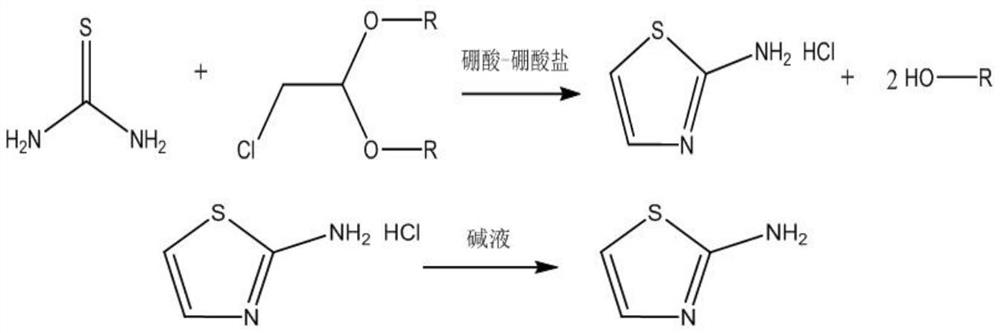
Preparation method of 2-aminothiazole
An aminothiazole, boric acid technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problems of poor color, low product yield and purity, etc. Achieve the effect of reducing side reactions, improving product yield and purity, and avoiding discoloration or even deterioration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A preparation method of 2-aminothiazole, comprising the steps:
[0042]S1. Mix 6.51g boric acid (99%) and 21.17g sodium tetraborate (99%) with a mortar and grind to powder to prepare a composite catalyst,
[0043] The prepared catalyst, 176.33g of thiourea (99%) and 265g of chloroacetaldehyde dimethyl acetal (98%) were added to a 500mL three-necked round-bottomed flask with magnetic stirring, connected with a thermometer and a condenser, and the reaction temperature was controlled at 90°C for 8h, to obtain the reaction product;
[0044] S2. connect underpressure distillation apparatus after the reaction finishes, set 65 ℃ of underpressure distillations to distill out by-product methanol, to distillate without distillate distillation, the reaction flask is moved in the freezing tank, and 145g 25% ammonia water is added dropwise while freezing, dripping The process temperature is kept below 40 °C, and a large number of white crystals (such as figure 1 After the dropwise...
Embodiment 2
[0048] A preparation method of 2-aminothiazole, comprising the steps:
[0049] S1. Mix 6.51 g of boric acid (99%) and 14.51 g of sodium metaborate tetrahydrate (99%) with a mortar and grind to powder to obtain a composite catalyst,
[0050] The prepared catalyst, 192.35g thiourea (99%) and 265g chloroacetaldehyde dimethyl acetal (98%) were added to a 500mL three-necked round bottom flask with magnetic stirring, connected with a thermometer and a condenser, and the reaction temperature was controlled at 90°C for 8h.
[0051] S2. connect underpressure distillation apparatus after the reaction finishes, set 65 ℃ of underpressure distillations to distill out by-product methanol, to distillate without distillate distillation, the reaction flask is moved in the freezing tank, and 145g 25% ammonia water is added dropwise while freezing, dripping The process temperature was kept below 40°C, a large number of white crystals were precipitated during the dropwise addition, pH=9.5 after t...
Embodiment 3
[0055] A preparation method of 2-aminothiazole, comprising the steps:
[0056] S1. Mix 5.21g of boric acid (99%) and 25.42g of sodium tetraborate (99%) with a mortar and grind to powder to obtain a composite catalyst. The obtained catalyst, 176.33g of thiourea (99%) and 324.67g of Chloroacetaldehyde diethyl acetal (98%) was added into a 500 mL three-necked round bottom flask with magnetic stirring, connected with a thermometer and a condenser, and the reaction temperature was controlled at 100°C for 8 hours.
[0057] S2. connect underpressure distillation apparatus after the reaction finishes, set 70 ℃ of underpressure distillations to distill out by-product ethanol, to distillate without distillate, the reaction flask is moved in the freezing tank, 290g 30% ammonia water is added dropwise while freezing, dripping The process temperature was kept below 40 °C, a large number of white crystals were precipitated during the dropwise addition, pH=10.1 after the dropwise addition of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com