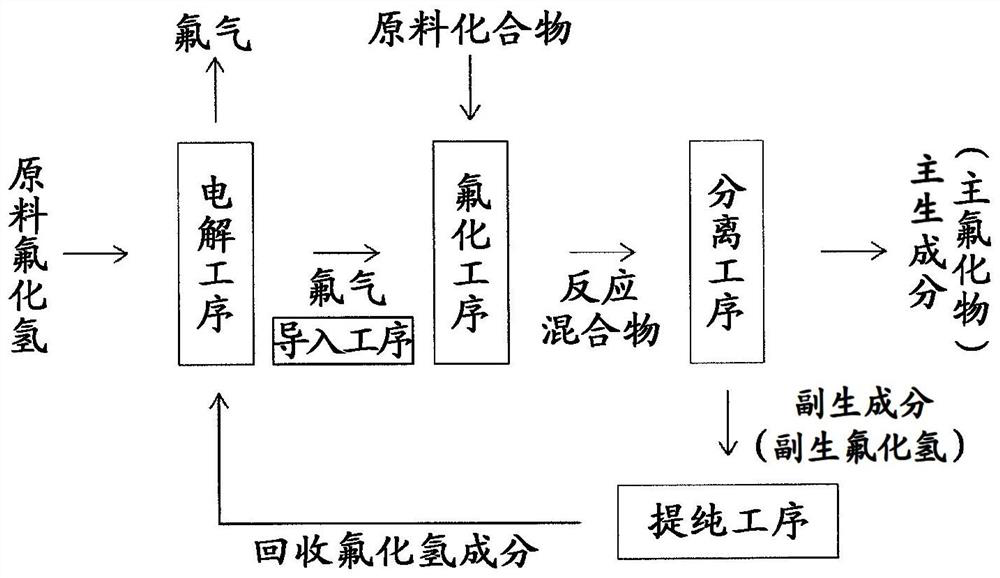
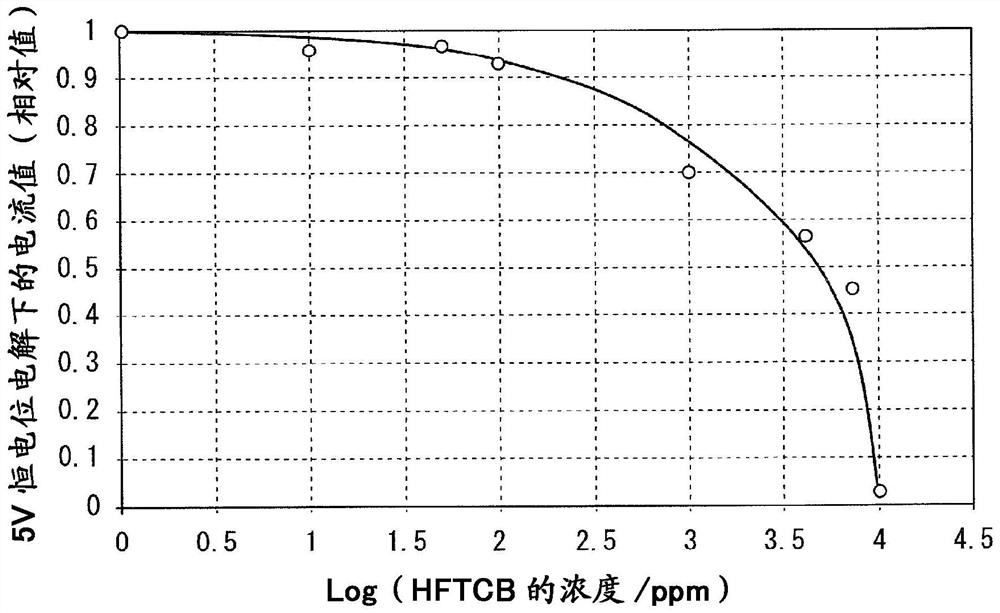
Fluorine gas production method
A manufacturing method and a fluorine gas technology, which are applied in the field of fluorine gas manufacturing, can solve the problem of high temperature of the reaction field, and achieve the effects of not easy performance deterioration and stable electrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] As a fluorination reaction, fluorine gas is reacted with 1,2,3,4-tetrachlorobutane (hereinafter referred to as "TCB") as a raw material compound to synthesize 1,1,2,3,4, which is the main fluoride compound. , 4-hexafluoro-1,2,3,4-tetrachlorobutane (boiling point 134 ° C) reaction.
[0084] In volume 4m 3 4700kg (2.8m 3 ) of the reaction solution obtained by adding TCB to the HFTCB solvent so that the concentration becomes 10% by mass, and using a stirrer with stirring blades (6 flat turbines) while stirring at a rotation speed of 70 rpm, A fluorine-containing gas diluted with nitrogen gas was supplied so that the fluorine gas concentration was 30% by volume, and fluorination of TCB was performed at a reaction temperature of 70°C.
[0085] The fluorine-containing gas was supplied into the reaction solution from the fluorine gas supply port provided in the reactor below the stirrer, and the supply rate of the fluorine-containing gas was 277 L / min (0° C., converted to 0 ...
Embodiment 2
[0096] Constant-current electrolysis was performed in the same manner as in Example 1, except that the B liquid of Example 1 was used as hydrogen fluoride replenished to the electrolytic cell. The constant current electrolysis was started, and 39g of liquid B was replenished every 100 hours. During the 1000 hours of constant current electrolysis, a total of 390g of hydrogen fluoride was added, but the cell voltage only rose to 7.2V, and the amount of fluorine gas produced remained unchanged.
Embodiment 3
[0101] As a fluorination reaction, fluorine gas is reacted with 1,1,2,2-tetrafluoroethane (hereinafter referred to as "TFE") as a raw material compound, and perfluoroethane (hereinafter referred to as "TFE") which is the main fluoride is synthesized by gas phase reaction. denoted as "PFE").
[0102] Fluorine gas and TFE were supplied to a cylindrical reactor with an inner diameter of 50 cm, a length of 8 m, and a capacity of 1570 L, and the temperature of the reactor was controlled to be 350° C., and the reaction was carried out at a reaction pressure of 0.2 MPaG (gauge pressure). In addition, fluorine gas is supplied after being diluted with PFE to a concentration of 4% by volume, and the supply amount of fluorine gas is 30Nm 3 . In addition, TFE is supplied after being diluted with PFE to a concentration of 2% by volume, and the supply amount of TFE is 15 Nm 3 .
[0103] In the separation process, the gas discharged from the outlet of the reactor is cooled to -45°C, the u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com