Synthesis method of eltrombopag
A synthetic method, the technology of hydrazine hydrate, which is applied in the design of organic synthesis routes and the preparation of raw materials and intermediates, can solve the problems of low yield, difficult protection, expensive raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
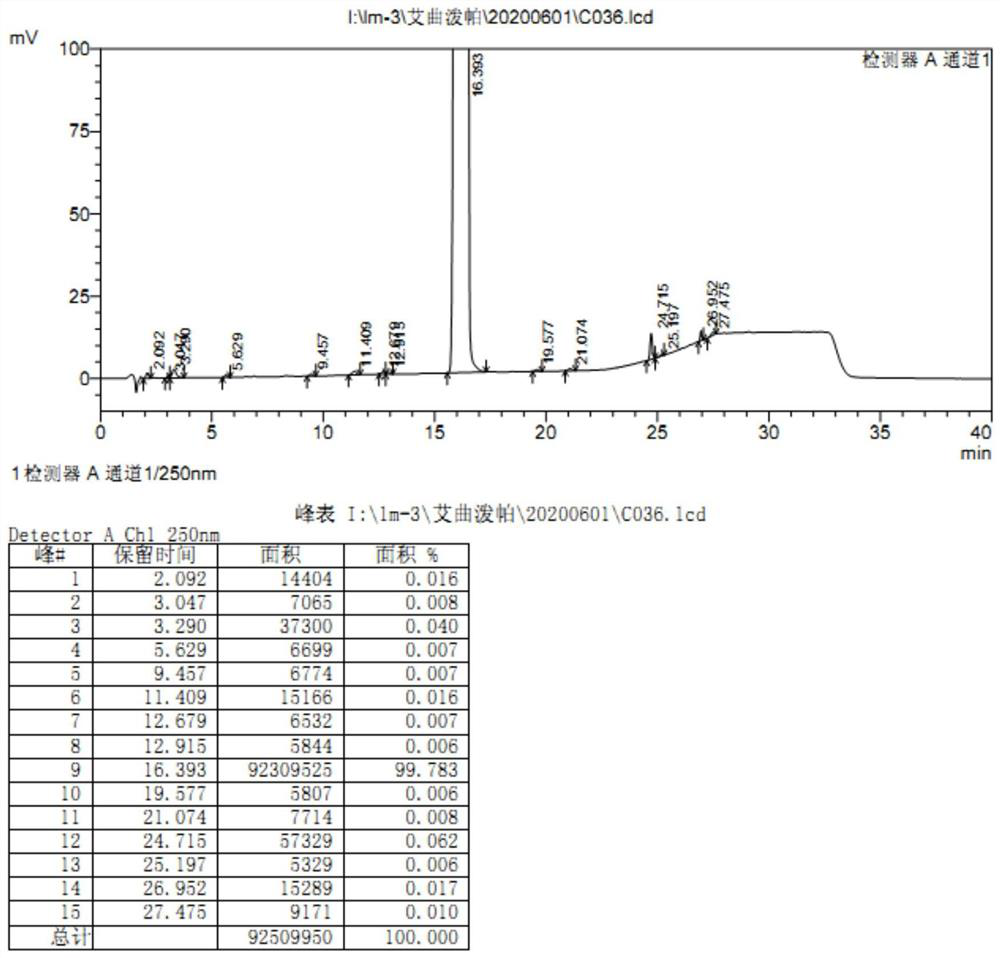
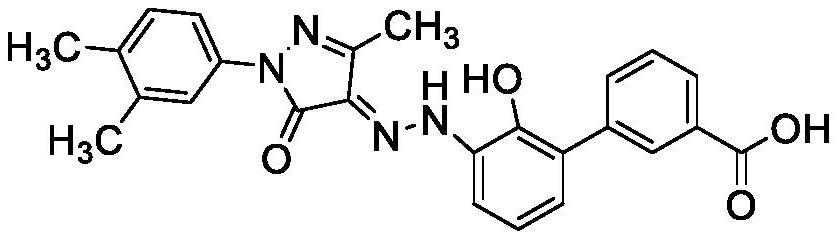
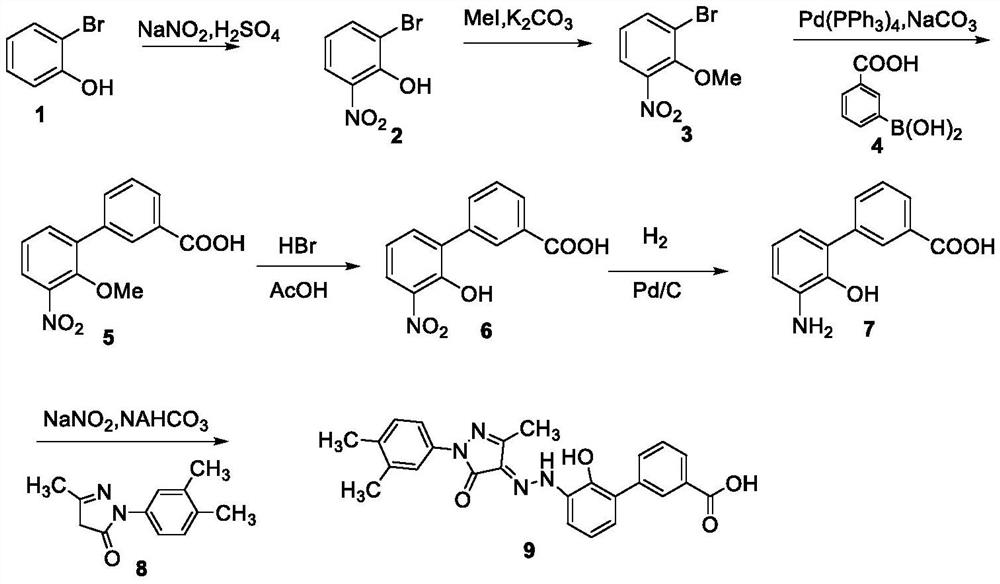
Image
Examples
Embodiment 1
[0027] Embodiment 1) the preparation of 3H-benzoxazol-2-ketone
[0028]
[0029] Dissolve 10.9 g of o-aminophenol in 200 ml of tetrahydrofuran, then add 16.3 g of N,N-carbonyl-diimidazole, heat up to reflux after adding, keep the temperature for 2 hours, then cool down to room temperature, quench the reaction with dilute hydrochloric acid, and depressurize After concentration, the residue was extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, and then concentrated under reduced pressure to obtain 12.2 g of 3H-benzoxazol-2-one with a yield of 90.4%.
Embodiment 2
[0030] Embodiment 2) the preparation of 3H-benzoxazol-2-ketone
[0031]
[0032] Dissolve 10.9 g of o-aminophenol in 200 ml of acetonitrile, then add 16.3 g of N,N-carbonyl-diimidazole, heat up to reflux after adding, keep the temperature for 2 hours, then cool down to room temperature, quench the reaction with dilute hydrochloric acid, and depressurize After concentration, the residue was extracted with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, and then concentrated under reduced pressure to obtain 11.5 g of 3H-benzoxazol-2-one with a yield of 85.2%.
Embodiment 3
[0033] Embodiment 3) the preparation of 7-bromo-3H-benzoxazol-2-ketone
[0034]
[0035] Add 250ml of glacial acetic acid and 250ml of water to 13.5g of 3H-benzoxazol-2-one, stir to dissolve, cool down to 0-5°C, slowly add 16.5g of liquid bromine dropwise under temperature control, stir overnight at room temperature, filter, The filter cake was washed with water and dried to obtain 13.3 g of 2-nitro-6-bromophenol with a yield of 62%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com