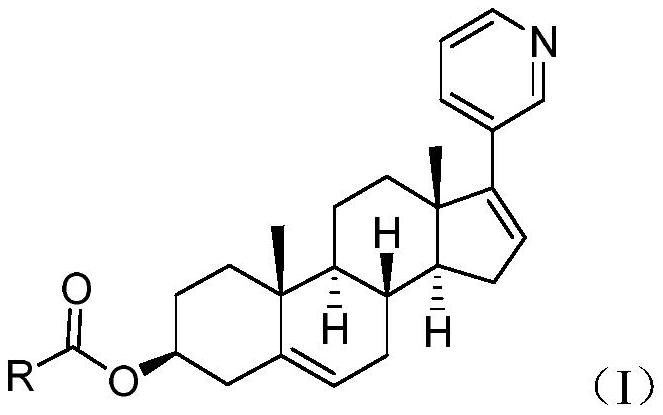
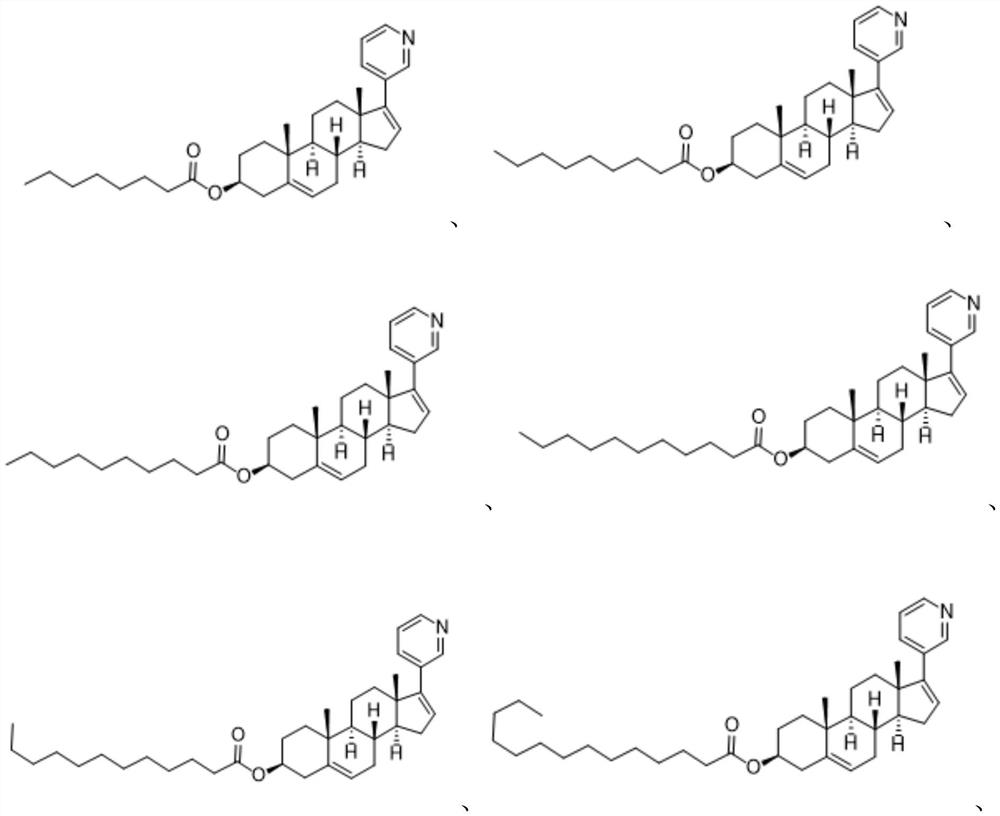
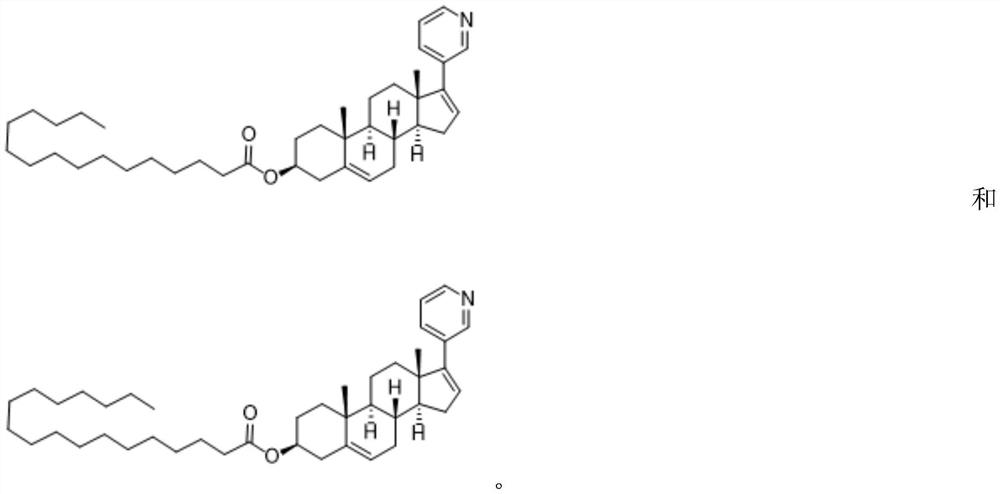
Abiraterone ester derivatives as well as preparation method and application thereof
A technology for drugs and compounds, applied in the field of medicine, can solve the problems of insufficient oral bioavailability, poor oral absorption, large oral dose, etc., and achieve the effect of improving oral bioavailability, high lipid solubility, and reducing oral dose.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] [(3S,8R,9S,10R,13S,14S)-10,13-Dimethyl-17-(3-pyridyl)-2,3,4,7,8,9,11,12,14, Synthesis of 15-Decahydro-1H-Cyclopenta[a]phenanthrene-3-yl]octanoate (Compound 1)
[0047]
[0048] Add 2.16g of n-octanoic acid and 10g of N,N-dimethylformamide into a 25mL single-necked bottle, stir to dissolve, add 1.9g of diisopropylcarbodiimide, and stir for 10min to obtain an active ester solution. Take another 100mL three-neck bottle, add 20g of N,N-dimethylformamide, add 3.5g of abiraterone under stirring, add the aforementioned active ester solution dropwise to the obtained suspension, and add 1.84g of dimethicone Aminopyridine, stirred at room temperature. After the reaction was completed, the reaction solution was poured into 200 mL of water, and extracted three times with ethyl acetate. The organic phases were combined, washed three times with saturated aqueous sodium chloride solution, and then dried with anhydrous sodium sulfate. The crude product obtained after concentratio...
Embodiment 2
[0052] [(3S,8R,9S,10R,13S,14S)-10,13-Dimethyl-17-(3-pyridyl)-2,3,4,7,8,9,11,12,14, Synthesis of 15-Decahydro-1H-Cyclopenta[a]phenanthrene-3-yl]nonanoate (Compound 2)
[0053]
[0054] Referring to the synthesis method of Example 1, using n-nonanoic acid to react with abiraterone, compound 2 was prepared with a yield of 37%.
[0055] 1 H NMR (400MHz, Chloroform-d) δ8.64 (d, J = 2.0Hz, 1H), 8.48 (dd, J = 4.8, 1.5Hz, 1H), 7.66 (dt, J = 7.9, 1.9Hz, 1H) ,7.24(dd, J=7.9,4.8Hz,1H),6.01(dd,J=3.0,1.7Hz,1H),5.44(d,J=5.1Hz,1H),4.65(tdd,J=10.4,6.0 ,4.2Hz,1H),2.42–2.33(m,2H),2.33–2.25(m,3H),2.14–2.02(m,3H),1.94–1.42(m,12H),1.41–1.24(m,10H ),1.24–1.09(m,5H),1.07(s,3H),0.90(t,J=6.8Hz,3H).
[0056] MS(ESI)m / z=490.6[M+H] +
Embodiment 3
[0058] [(3S,8R,9S,10R,13S,14S)-10,13-Dimethyl-17-(3-pyridyl)-2,3,4,7,8,9,11,12,14, Synthesis of 15-Decahydro-1H-Cyclopenta[a]phenanthrene-3-yl]decanoate (Compound 3)
[0059]
[0060] Referring to the synthesis method of Example 1, using n-capric acid to react with abiraterone, compound 3 was prepared with a yield of 48%.
[0061] 1 H NMR (400MHz, Chloroform-d) δ8.64 (d, J = 1.9Hz, 1H), 8.48 (dd, J = 4.8, 1.4Hz, 1H), 7.67 (dt, J = 7.9, 1.8Hz, 1H) ,7.24(dd,J=7.9,4.8Hz,1H),6.01(dd,J=3.0,1.7Hz,1H),5.44(d,J=5.0Hz,1H),4.71–4.59(m,1H), 2.39–2.33(m,2H),2.33–2.25(m,3H),2.14–2.02(m,3H),1.94–1.85(m,2H),1.84–1.42(m,11H),1.40–1.24(m ,12H),1.24–1.09(m,5H),1.07(s,3H),0.90(t,J=6.8Hz,3H).
[0062] MS(ESI)m / z=504.6[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com