SARS-CoV-2 mutant strain S protein and subunit vaccine thereof
A subunit vaccine, coronavirus technology, applied in the field of new coronavirus mutant S protein and its subunit vaccine, can solve the problem of reduced neutralizing activity of Delta mutant strain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
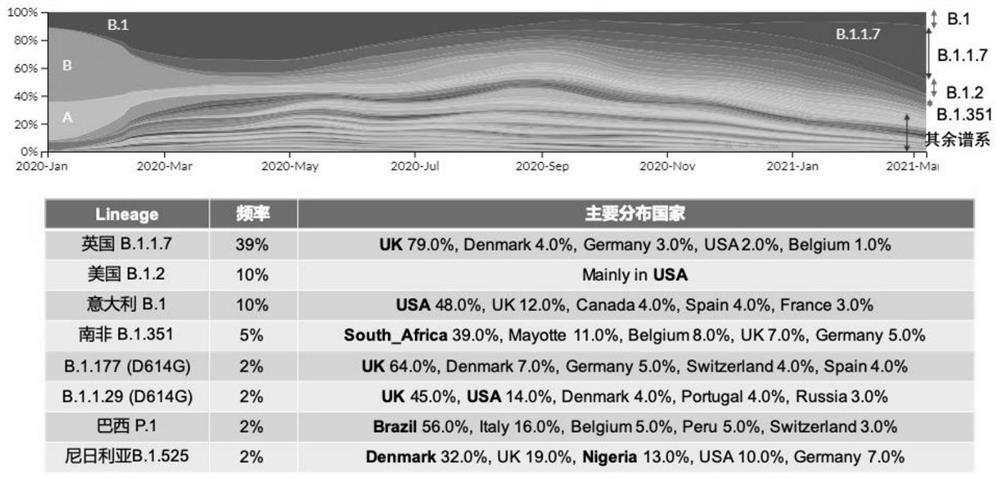
[0078] Example 1. Prediction of consensus sequences Due to the high mutagenicity of SARS-CoV-2, especially the key receptor binding domain (RBD) of the mutation site on the S protein, its binding ability to the receptor ACE2 may be enhanced, increasing the The transmissibility or pathogenicity of the virus; at the same time, mutations may change the key epitopes of the antigen, reducing the affinity of neutralizing antibodies that have been screened, or reducing the protective effect of vaccines and neutralizing antibodies. At present, SARS-CoV-2 has evolved Multiple types of mutations ( figure 1 ). For these mutants, we adopted four different strategies to compare the consensus sequences of mutants by software prediction.
[0079] 1. Strategy 1:
[0080] We downloaded 2674 (de-duplicated) 2019-nCoV S protein sequences from the NCBI database (as of February 28, 2021), and constructed a phylogenetic tree of these S proteins by neighbor-joining method using MEGA 7.0 software (...
Embodiment 2
[0090] Embodiment 2. Construction and expression optimization of recombinant S protein vector
[0091] 1. Construction of the S protein gene expressed in the supernatant of mammalian cells
[0092] The schematic diagram of the construction of the S protein expression gene of the present invention is shown in Image 6 and Figure 7 .
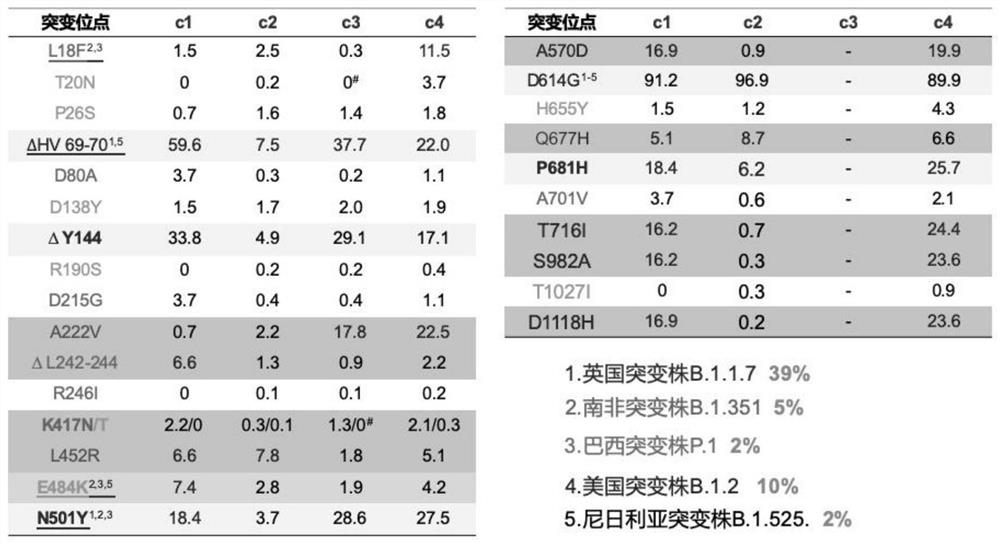
[0093] Image 6 : Consensus sequence S6 schema diagram. This sequence contains deletions of amino acids 69-70 and 144, and contains E484K, N501Y, D614G, P681H mutations. At the same time, in order to maintain the integrity of the recombinant S protein, the Furin cleavage site was mutated to GSAS, GS combination, or (GGGS)n or (GGGGS)n or (G)n, with the value of n, the length of the linker connecting S1 and S2 also change. To ensure that the recombinant S protein is secreted and expressed in the form of a native trimer, the C-terminal transmembrane domain was replaced with a T4 phage Fibritin trimer motif or a GCN4 multimer formation motif. ...
Embodiment 3
[0102] Example 3. Immunization and effect identification of recombinant S6 / S15 protein trimer vaccine
[0103] 1. The immune process of mice
[0104] The mice used in this experiment were K18-hACE female mice, 6-8 weeks, 19-25 g, purchased from Jiangsu Jicui Yaokang Biotechnology Co., Ltd. All animal experiments were performed in the SPF laboratory. There were 12 mice in the S6 vaccine experimental group; 5 mice in the S15 vaccine experimental group; 12 mice in the prototype strain S vaccine experimental group; and 12 mice in the control group inoculated with PBS. Orbital blood was collected from all mice 14 days after the first injection of the vaccine and 28 days after the first injection (i.e., 14 days after the second injection). The mouse serum was taken to test serum neutralizing antibody titers.
[0105] 2. The serum of mice immunized with the vaccine of the present invention contains extremely high anti-S IgG
[0106] In order to verify that the designed vaccine can...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com