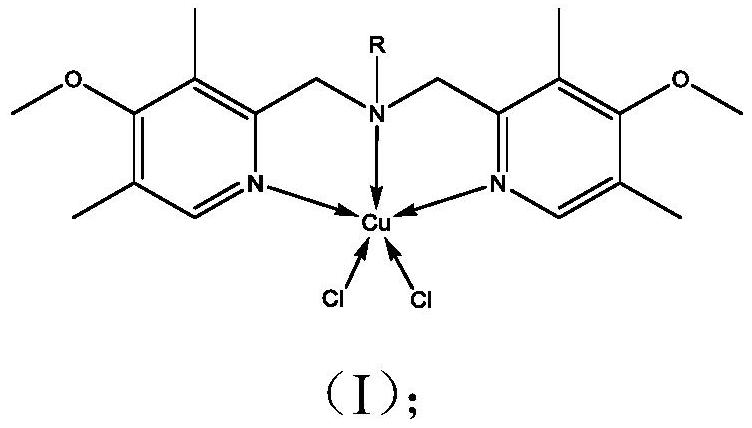
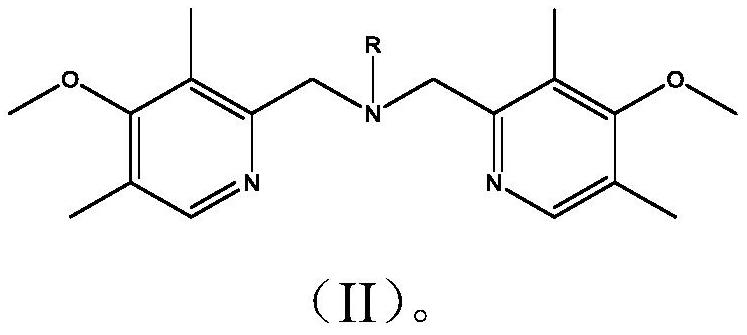
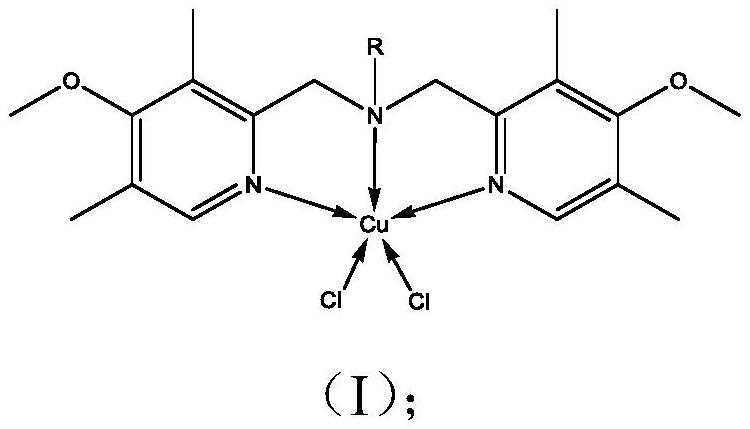
Bipyridine amine copper complex with anticancer activity as well as synthesis method and application of bipyridine amine copper complex
A technology of copper dipyridylamine and anticancer activity is applied in the field of anticancer metal complexes, which can solve the problems of the toxic and side effects of platinum anticancer drugs, and achieve the effects of good inhibitory effect, low cost and good anticancer effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Bipyridylamine copper complex [Cu(BPMA-n-C 18 h 37 )Cl 2 ]Synthesis
[0039] (1) Ligand BPMA-n-C 18 h 37 synthesis:
[0040] Add 13.3g 2-chloromethyl-4-methoxyl-3,5-lutidine hydrochloride, 8.09g n-octadecylamine and 0.762g hexadecyltributylphosphine bromide to a 250ml round In the bottom flask, add 105ml tetrahydrofuran to dissolve. Subsequently, 60 ml of 5 mol / L sodium hydroxide aqueous solution was added, and the mixture was stirred and reacted at 60° C. for 5 days. After the reaction was completed, the solution was transferred to a separatory funnel, the organic phase was separated and retained, and 30 ml of saturated sodium chloride solution was added for washing, and the process was repeated four times. Add desiccant anhydrous MgSO to the organic phase 4 Filter after drying. The filtrate was rotary evaporated to remove most of the solvent, and then placed in a vacuum drying oven to dry to constant weight to obtain a yellow oily substance, which was the tar...
Embodiment 2
[0045] The synthesis process is the same as in Example 1, the only difference being that n-octadecylamine in the ligand synthesis process is replaced by n-heptadecylamine.
[0046] After elemental analysis, the theoretical value: C, 61.07%; H, 8.64%; N, 6.10%. The measured value C, 61.23%; H, 8.52%; N, 6.39%; basically consistent with the theoretical value.
Embodiment 3
[0048] The synthesis process is the same as in Example 1, the only difference being that n-octadecylamine in the ligand synthesis process is replaced by n-hexadecylamine.
[0049] After elemental analysis, the theoretical value: C, 60.56%; H, 8.52%; N, 6.23%. The measured value C, 60.24%; H, 8.39%; N, 6.41%; basically consistent with the theoretical value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com