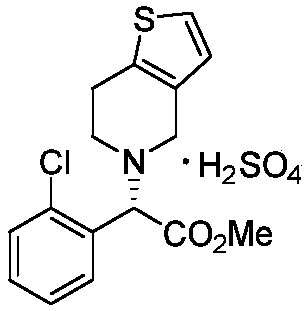
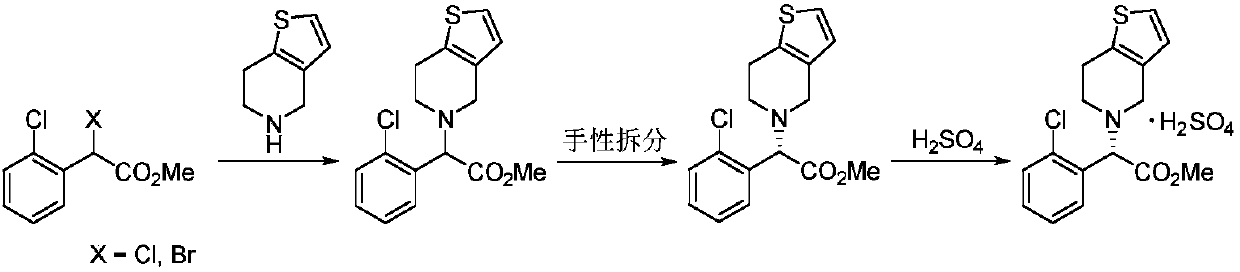
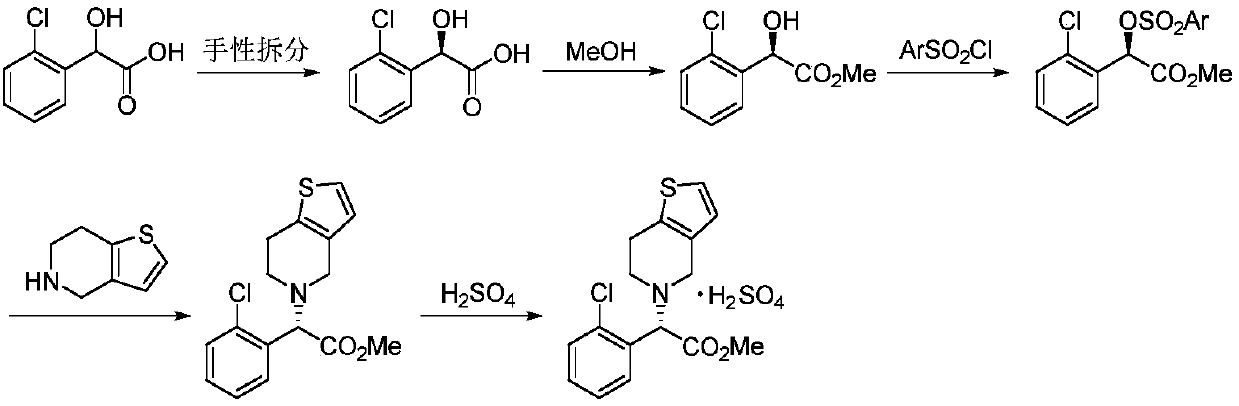
Novel method for synthesizing clopidogrel bisulphate and intermediate thereof
A technology of clopidogrel and a new method, which is applied in the field of synthesizing clopidogrel hydrogen sulfate and its intermediates, can solve the problems of non-compliance with the green development strategy, waste of configuration isomers, etc., and is beneficial to the industrial production of products, The effect of short synthetic route and high conversion number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] Catalyst preparation: [Ir(COD)Cl] 2 (1eq.), (R)-f-amphox (2eq.) were mixed in methanol, stirred at room temperature for 2.5 hours and then directly used for asymmetric hydrogenation catalytic reaction;
[0071] [Rh(COD)Cl] 2 (1eq.), (R)-BINAP (2.2eq.) were mixed in methanol, stirred at room temperature for 2 hours and then directly used for asymmetric hydrogenation catalytic reaction;
Embodiment 1
[0072] Embodiment 1 The preparation method of the chiral secondary amine compound shown in a kind of formula (II)
[0073]
[0074] Add 12.3g (0.04mol) of imine (I) into a 200mL stainless steel autoclave, then add 80mL of methanol and stir to dissolve, add 652mg of cesium carbonate (5mol%, 0.002mol), and then add (R) with a concentration of 0.01mol / L -f-amphox / [Ir(COD)Cl] 2 Catalyst 0.8mL, the molar ratio of imine (I) and catalyst is 5000 / 1. 4 MPa of hydrogen gas was introduced into the reactor, the reaction was stirred at 25°C for 24 hours, and the solvent was distilled off. After separation and purification by column chromatography, 12.1 g of chiral secondary amine (II) was obtained, with a yield of 97.9% and an enantiomeric excess of more than 99%.
Embodiment 2
[0075] Embodiment 2 The preparation method of the chiral secondary amine compound shown in a kind of formula (II)
[0076]
[0077] Add 12.3g (0.04mol) of imine (I) into a 200mL stainless steel autoclave, then add 80mL of methanol and stir to dissolve, add 14mg(R)-BINAP-[Rh(COD)Cl] 2 The molar ratio of catalyst to substrate catalyst is 5000 / 1. 4 MPa of hydrogen gas was introduced into the reaction vessel, the reaction was stirred at 25°C for 24 hours, and the solvent was distilled off. After separation and purification by column chromatography, 11.9 g of chiral secondary amine (II) was obtained, with a yield of 96.3% and an enantiomeric excess of more than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com