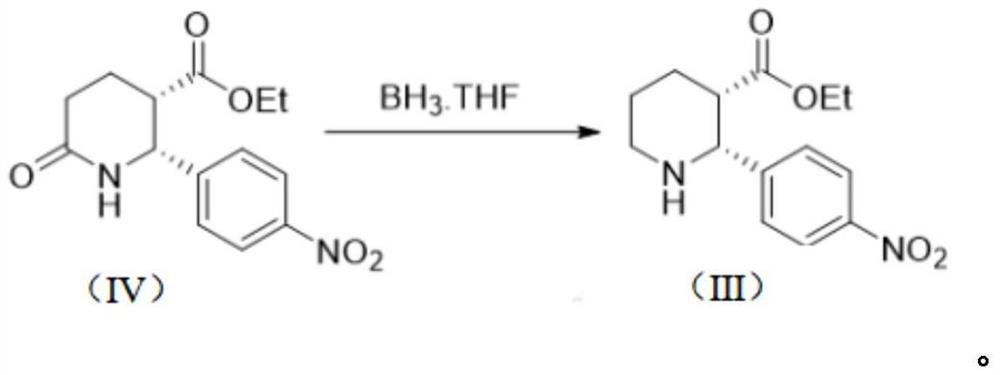
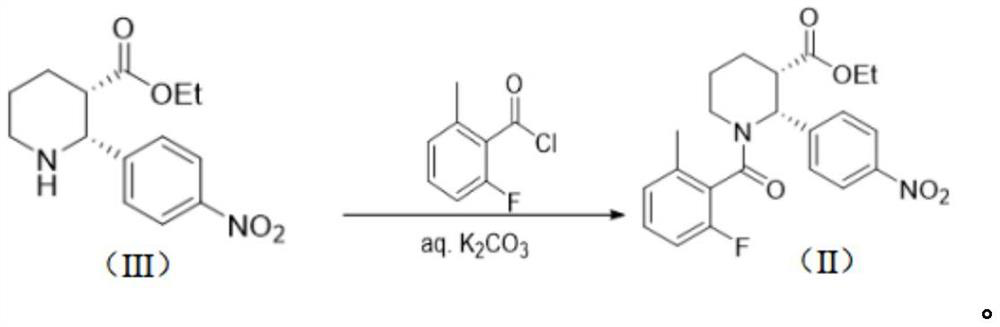
Preparation method of Avopan and intermediate thereof
A technology of body and compound, applied in the field of organic synthesis, can solve the problems of reduced purity and yield of target product, high cost, unsuitable for industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
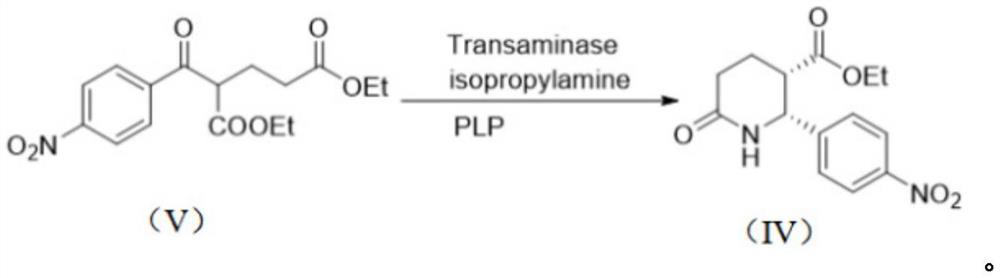
[0042] Embodiment 1-5 A kind of preparation method of the compound shown in Avacopan intermediate formula (IV)
[0043] Basic embodiment: the compound shown in the formula (IV) is obtained by the transamination reaction of the compound shown in the formula (V) and isopropylamine or its salt, and the transamination reaction is catalyzed by ω-transaminase and pyridoxal phosphate next.
[0044]
[0045] In a 250mL three-necked flask, add the compound 2-(4-nitrobenzoyl)valeric acid diethyl ester (33.7g, 0.1mol) shown in formula (Ⅴ), 5% DMSO (1L), and stir to dissolve; Isopropylamine salt (19.0g, 0.2mol), Na 2 HPO 4 and Na 2 HPO 4 buffer solution (pH=7.5, 150mL); control the reaction temperature at 60-70°C, add ω-transaminase (1g) and pyridoxal phosphate (0.247g, 1mmol), and stir at 10°C for 2 days. Extract with 200mL ethyl acetate, repeat the extraction twice; concentrate the organic phase to about 200mL, heat up to 70-80°C, add n-heptane (200mL) dropwise; naturally cool t...
Embodiment 2
[0047] The difference between this embodiment and Example 1 is that the molar ratio of the compound shown in formula (V) to diisopropylamine salt is 1:4, and the molar ratio of the compound shown in formula (V) to pyridoxal phosphate is 200:1 , the mass ratio of the compound represented by formula (Ⅴ) to ω-transaminase is 100:1, the reaction temperature is 30-40° C., and the rest are the same as in Example 1.
Embodiment 3
[0049]The difference between this embodiment and Example 1 is that the molar ratio of the compound shown in formula (V) to diisopropylamine salt is 1:1, and the molar ratio of the compound shown in formula (V) to pyridoxal phosphate is 150:1 , the mass ratio of the compound represented by formula (Ⅴ) to ω-transaminase is 20:1, the reaction temperature is 10-20° C., and the rest are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com