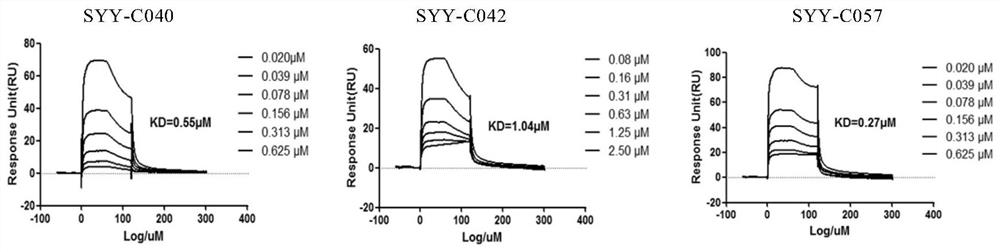
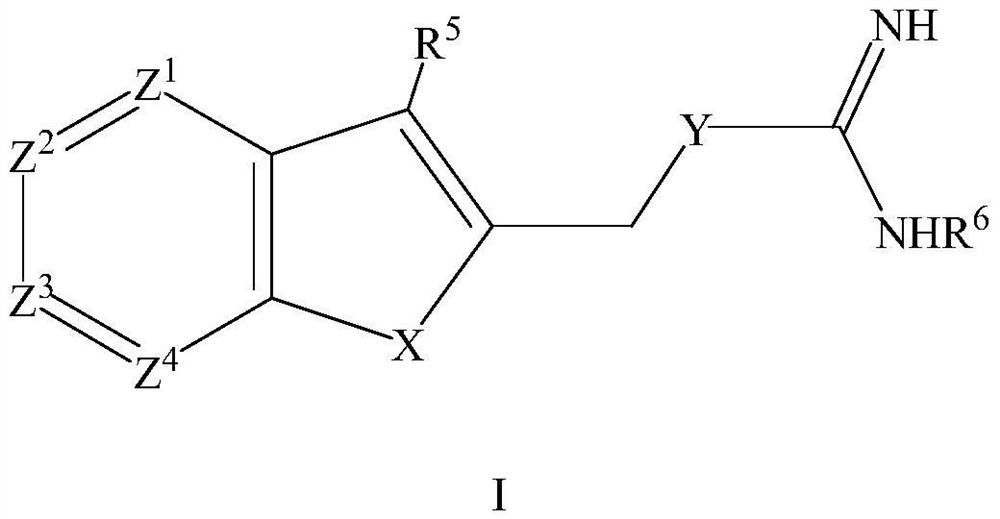
Compound for treating thrombotic diseases
A compound and solvate technology, which is applied in the field of compounds for the treatment of thrombotic diseases, can solve the problems of low utilization efficiency, poor drugability, and poor permeability of simulated peptides.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0236] Embodiment 1: the preparation of compound SYY-C001
[0237]
[0238] step 1:
[0239] Under mechanical stirring, 2-methoxy-3-chloro-5-nitropyridine (37.0g, 196.2mmol) was added in ethanol (500ml), iron powder (40.0g, 716.2mmol) was added at room temperature, ammonium chloride (40.0 g, 747.8 mmol) and water (100 ml), heated to 90° C. for 5 hours, and TLC showed that the reaction was complete. The reaction liquid was filtered, and the filtrate was concentrated to about 150 mL, added ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and the crude product was subjected to silica gel column chromatography to obtain gray solid C001-1 (30.5 g, yield 98%).
[0240] Step 2:
[0241] C001-1 (30.5g, 192.3mmol) was dissolved in acetonitrile (400ml), cooled to 0°C, NIS (47.1g, 209.4mmol, dissolved in 300ml of acetonitrile) was added dropwise, and maintained at 0-2°C for 1 hour After dropping, TLC monitors (a small amount of raw mate...
Embodiment 2
[0262] Embodiment 2: Preparation of intermediates C-IN-002 and C-IN-003
[0263]
[0264] step 1:
[0265] N 2 Under protection, the raw material 2-amino-5-bromopyridine (30.0g, 173.40mmol) was dissolved in DMF (200mL), and trifluoroacetic acid (14.2mL, 191.17mmol) and NIS (43.0g, 191.13mmol) were added at room temperature , heated to 50° C. and stirred for 3 hours, TLC showed that the starting material was completely consumed. The reaction solution was cooled to room temperature, the reaction solution was slowly poured into water, extracted with ethyl acetate, the organic phases were combined, and 10% NaS 2 SO 3 solution, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to give IN-002-1 (45g) as a yellow solid.
[0266] Step 2:
[0267] N 2 Under protection, IN-002-1 (2.0g, 6.69mmol) and Et 3 N (1.2mL, 8.63mmol) was dissolved in THF (30mL), and trimethylsilylpropyne (2.0mL, 13.51mmol) was added in portions at room temperature, and P...
Embodiment 3
[0276] Embodiment 3: the preparation of compound SYY-C002
[0277]
[0278] step 1:
[0279] N 2 Under protection, C-IN-002 (1.2g, 8.21mmol) was dissolved in DMF (20mL), and NBS (1.46g, 8.20mmol) was added under an ice-water bath. After 10 minutes of reaction, TLC showed that the raw material was completely reacted. Add water to the reaction solution, extract with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate. The crude product is subjected to silica gel column chromatography to obtain yellow solid C002-1 (1.5 g, yield 81%). 1 H NMR (400MHz, CDCl 3 )δ2.48(s, 3H), 3.83(s, 3H), 7.10(dd, J=7.8, 4.8Hz, 1H), 7.76(dd, J=7.8, 1.5Hz, 1H), 8.29(dd, J =4.8, 1.5Hz, 1H).
[0280] Step 2:
[0281] N 2 Under protection, C002-1 (1.5g, 6.66mmol) was dissolved in THF (30mL), cooled to -65°C, and n-BuLi (2.8mL, 7.00mmol, 2.5M) was slowly added dropwise, and the addition was completed in - After stirring at 65°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com