Kit for tigecycline drug sensitivity detection and method for tigecycline drug sensitivity detection by using same
A technology of tigecycline and kits, applied in biochemical equipment and methods, microbial measurement/testing, Raman scattering, etc., can solve the problems of complex operation, difficult standardization, and high MIC value, and achieve simple and accurate operation The effect of high precision and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
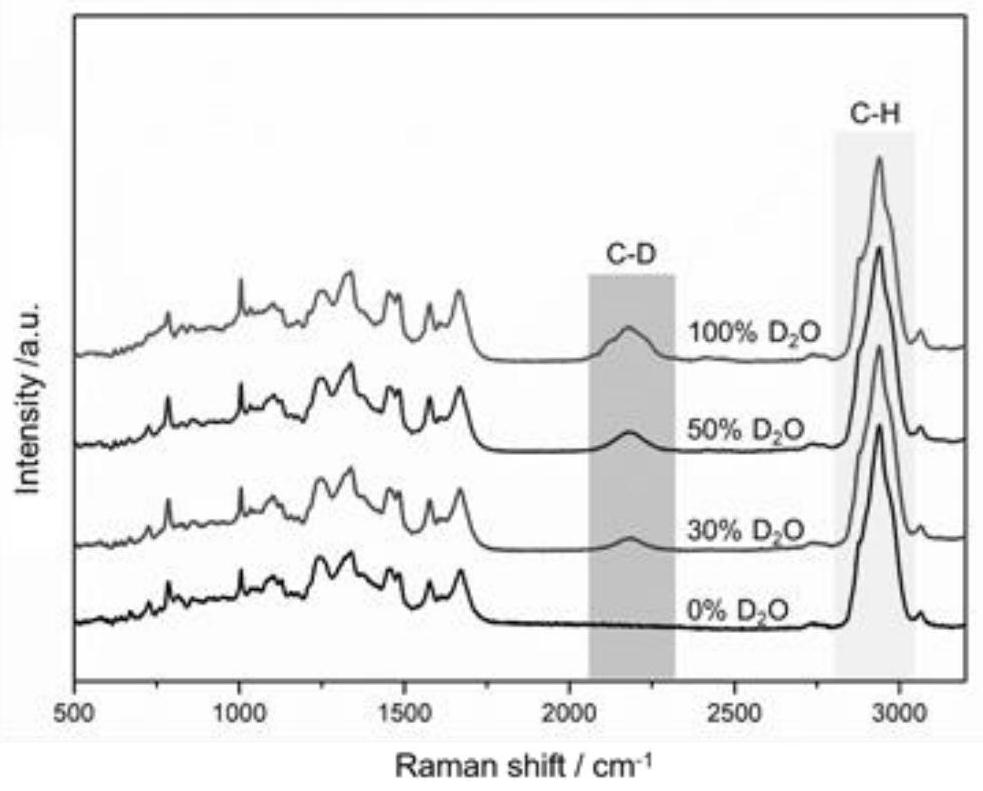
[0039] Embodiment 1, research on optimum heavy water concentration
[0040] 1. Experimental Preparation
[0041] 1) Culture medium preparation
[0042] Preparation of CAMHB medium: Weigh 2.1g of MUELLER-HINTON BROTH (M-H broth medium, referred to as MH medium) powder and dissolve it in 100mL ddH 2 O, add Ca 2+ solution and Mg 2+ solution, so that the final concentrations were 25mg / L and 12.5mg / L, respectively, and sterilized under high temperature and high pressure at 121°C for 15min.
[0043] 2) Preparation of bacterial suspension
[0044] The frozen standard Acinetobacter baumannii strain (Acinetobacter baumannii, ATCC19606) was inoculated into Columbia blood plates and cultured overnight in an incubator at 35°C±2°C. Pick a single colony and inoculate it into CAMHB medium, incubate at 35°C±2°C, and adjust to 0.5 McFarland turbidity.
[0045] 2. Effect of heavy water concentration on strain CD-ratio
[0046] Add the ATCC19606 bacterial solution to CAMHB medium containi...
Embodiment 2
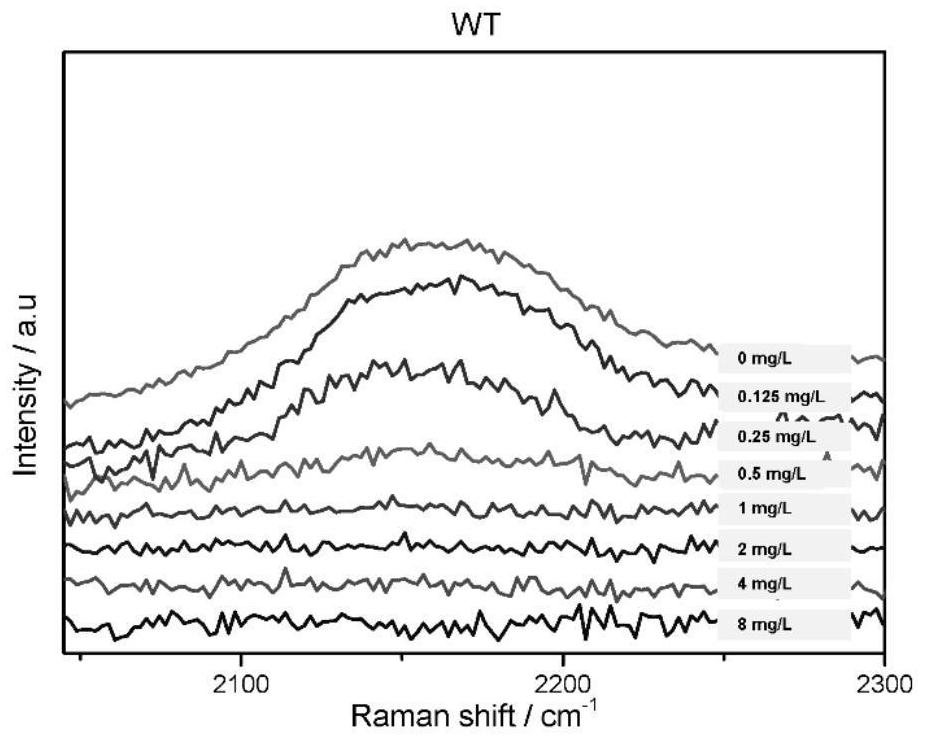
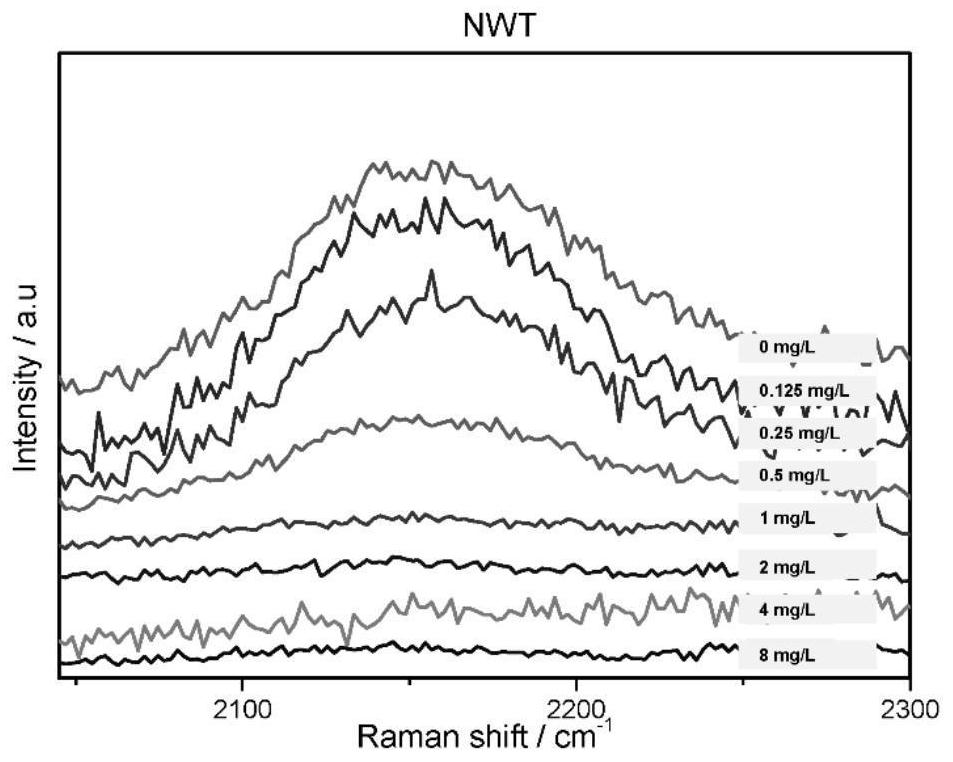
[0055] Embodiment 2, drug susceptibility test of tigecycline
[0056] 1. Experimental Preparation
[0057] 1) Culture medium preparation
[0058] 1×CAMHB medium: Weigh 2.1g of MUELLER-HINTON BROTH powder, dissolve in 100mL ddH 2 O, add Ca 2+ solution and Mg 2+ Solution, so that the final concentration is 25mg / L, 12.5mg / L respectively, sterilized under high temperature and high pressure at 121℃ for 15min;
[0059] 2×CAMHB medium: Weigh 4.2g of MUELLER-HINTON BROTH powder, dissolve in 100mL ddH 2 O, add Ca 2+ solution and Mg 2+ solution, so that the final concentrations were 50mg / L and 25mg / L, respectively, and sterilized under high temperature and high pressure at 121°C for 15min.
[0060] 2) Drug preparation
[0061] Tigecycline (Tigecycline, Tgc) stock solution: use ddH 2 O Prepare tigecycline mother solution with a concentration of 16 mg / mL, aliquot into centrifuge tubes, wrap them in tin foil, and store at -80°C. (Note: The whole process needs to be operated and s...
Embodiment 3
[0080] Embodiment 3, tigecycline susceptibility detection kit and application
[0081] 1. Kit composition
[0082] Table 1 Composition of the kit
[0083]
[0084] 2. Kit application
[0085] 1) Sample preparation: Pick a single colony on the plate of the clinical sample to be tested (pathogenic bacteria shown in Table 2, strain source references Zhang Z, Chen M, Yu Y, et al.Antimicrobial susceptibility among gram-positive and gram- negative blood-borne pathogens collected between 2012-2016as part of the Tigecycline Evaluation and Surveillance Trial[J]. Antimicrobial Resistance&Infection Control, 2018, 7(1).) Inoculated in CAMHB medium, incubated at 35℃±2℃, adjusted to 0.5 McFarland turbidity, made of bacteria suspension.
[0086] 2) Drug sensitivity plate preparation: add culture medium to the drug sensitivity plate to fully dissolve the tigecycline powder, and avoid light during the whole process.
[0087] 3) Inoculate the suspension of the bacteria to be tested in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com