Vanadium pentoxide nanobelt with hydrogen bond network as well as preparation and application thereof
A nanobelt and hydrogen bond technology, applied in the field of electrochemistry, can solve problems such as unsatisfactory electrochemical performance, specific capacity decay, hindering the kinetic process of electrochemical intercalation reaction, etc., to achieve improved electrochemical performance, stable cycle performance, The effect of excellent rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
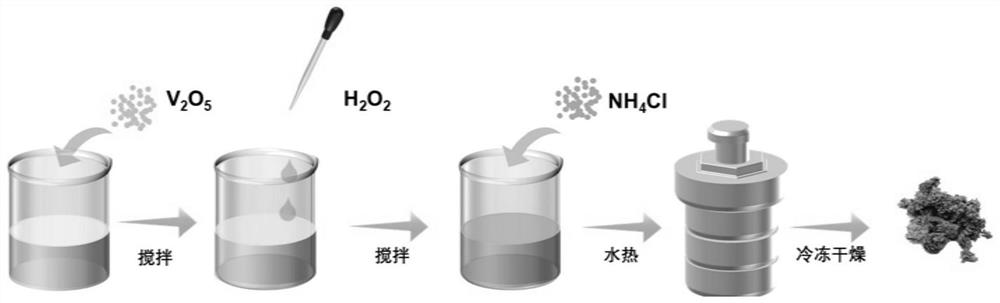
[0035] A V with a hydrogen bond network 2 o 5 The preparation method of the nanoribbon The preparation method comprises:
[0036] 1) Mix 3 mmol of commercial V 2 o 5 Dissolve the powder in 24mL of deionized water and stir in a beaker to form a uniformly dispersed yellow suspension;
[0037] 2) Add 250mmol H to the suspension obtained in step 1) 2 o 2 , stirring and dissolving to form a uniformly dispersed orange solution.
[0038] 3) Add different amounts of 2, 10, 30mmol NH to the solution obtained in step 2) 4 Cl salt, stirred evenly, moved into the reactor, hydrothermally reacted at 200°C for 48 hours, centrifuged and washed, and then freeze-dried to obtain V with hydrogen bond network with different ammonium ion concentrations. 2 o 5 nanobelt.
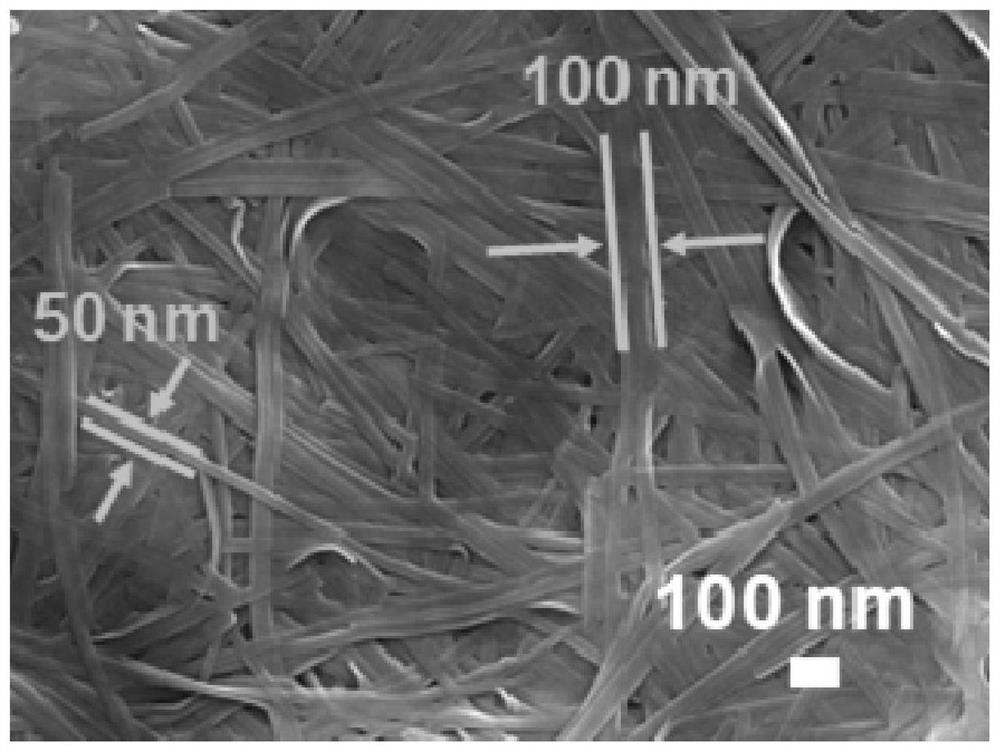
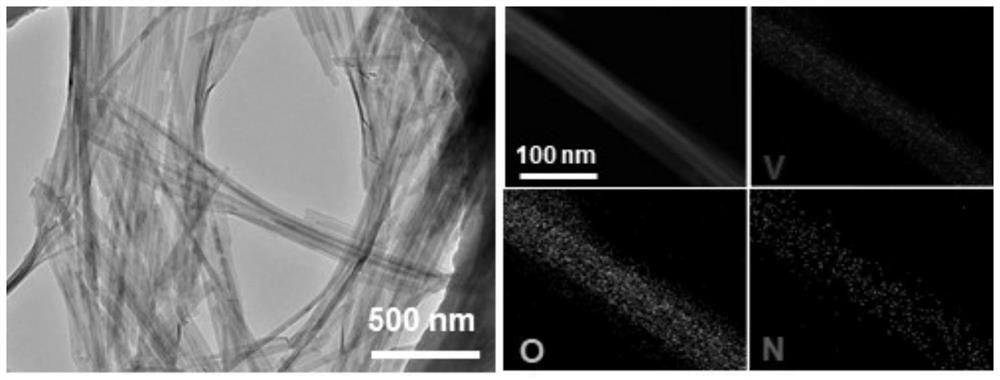
[0039] The scanning electron microscope picture of the product of this embodiment is shown in figure 2 As shown, it can be seen that compared to the original commercial block V 2 o 5 , The product obtained in Example 1...
Embodiment 2
[0047] A V with a hydrogen bond network 2 o 5 The preparation method of the nanoribbon The preparation method comprises:
[0048] 1) Mix 3 mmol of commercial V 2 o 5 Dissolve the powder in 40mL of deionized water and stir in a beaker to form a uniformly dispersed yellow suspension;
[0049] 2) Add 300mmol to the suspension obtained in step 1), stir and dissolve to form a uniformly dispersed orange solution.
[0050] 3) Add 10mmol NH in the solution obtained in step 2) 4 Cl, stirred evenly, moved into the reactor, hydrothermally reacted at 180°C for 48 hours, centrifuged and washed, and then freeze-dried to obtain V with a hydrogen bond network 2 o 5 nanobelt.
[0051] With the V with hydrogen bond network obtained in this example 2 o 5 Taking nanobelts as an example, the structural characterization and electrochemical performance improvement are similar to those in Example 1.
Embodiment 3
[0053] A V with a hydrogen bond network 2 o 5 The preparation method of the nanoribbon The preparation method comprises:
[0054] 1) Mix 3 mmol of commercial V 2 o 5 Dissolve the powder in 50mL deionized water and stir in a beaker to form a uniformly dispersed yellow suspension;
[0055] 2) Add 250ml H to the suspension obtained in step 1) 2 o 2 , stirring and dissolving to form a uniformly dispersed orange solution.
[0056] 3) Add 30mmol NH in the solution obtained in step 2) 4 Cl, stirred evenly, moved into the reactor, hydrothermally reacted at 200°C for 48 hours, centrifuged and washed, and then freeze-dried to obtain V with a hydrogen bond network 2 o 5 nanobelt.
[0057] With the V with hydrogen bond network obtained in this example 2 o 5 Taking nanobelts as an example, the structural characterization and electrochemical performance improvement are similar to those in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com