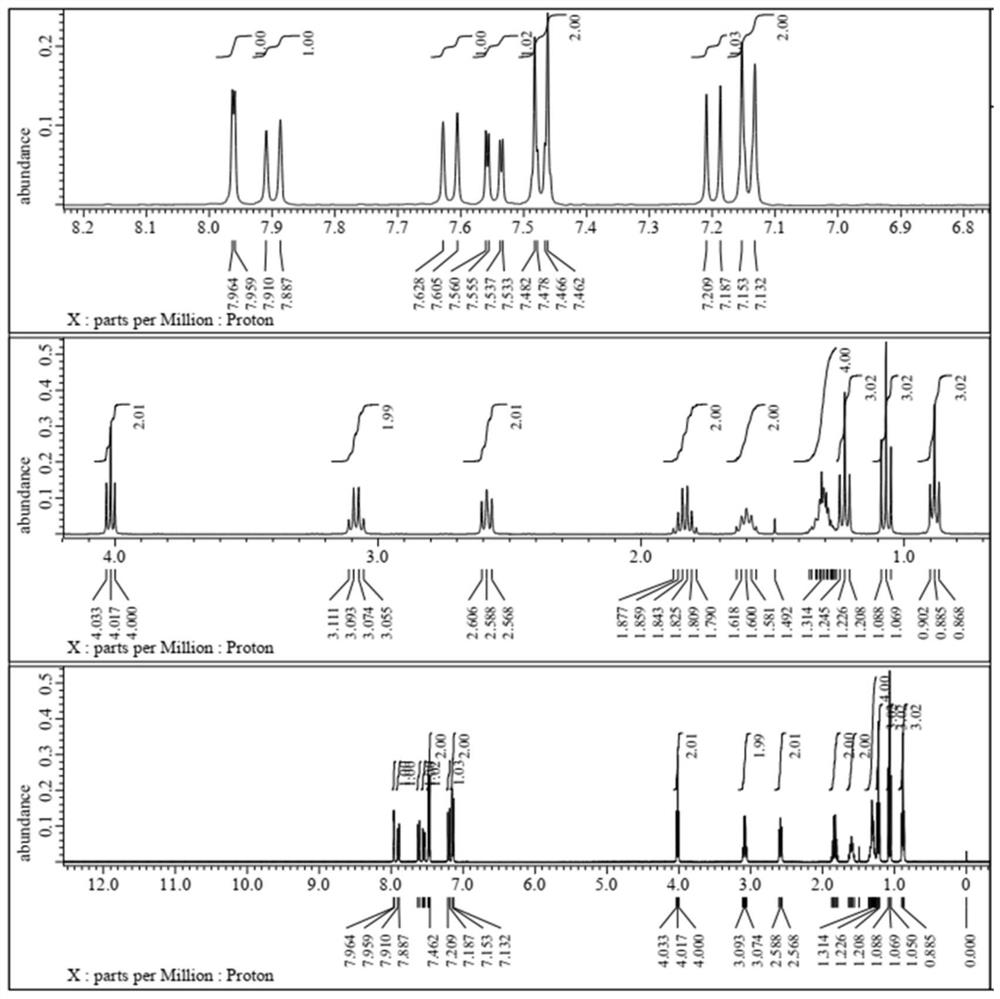
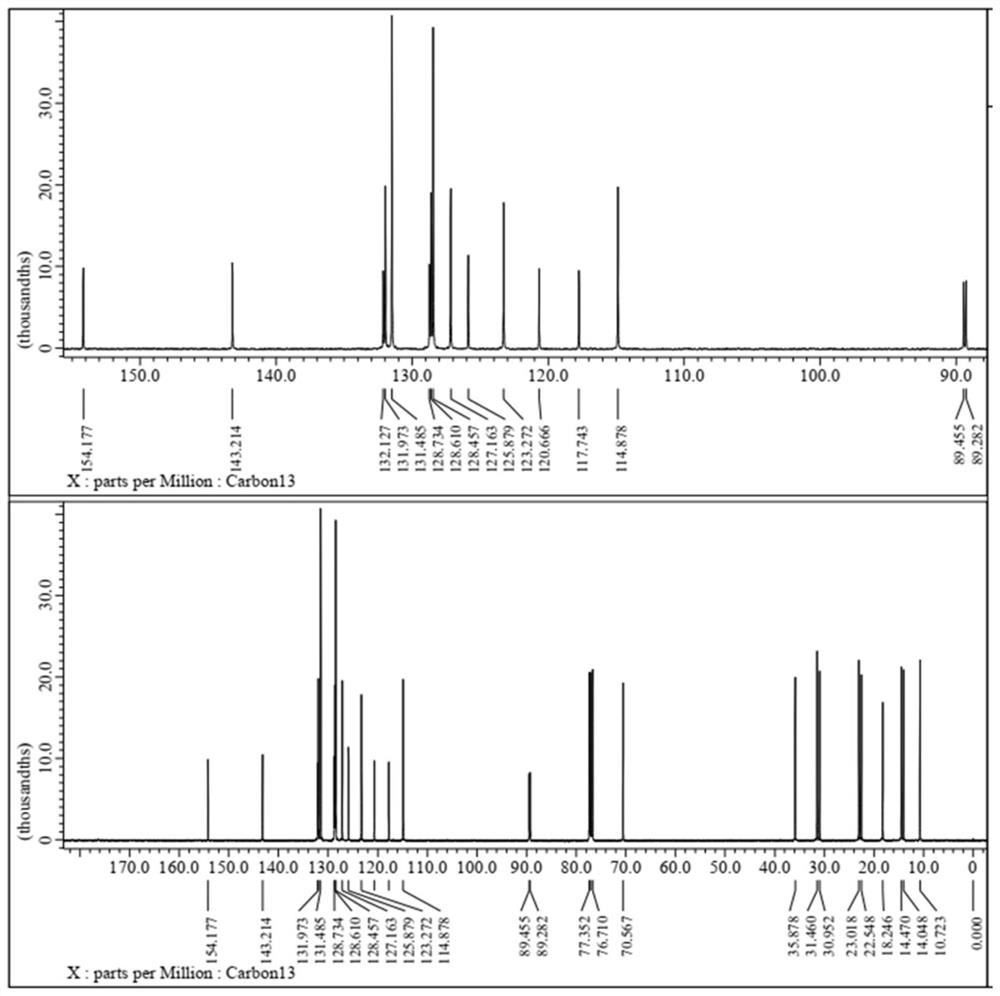
Single-naphthalene-containing liquid crystal monomer compound as well as preparation method and application thereof
A technology of liquid crystal monomers and compounds, applied in chemical instruments and methods, liquid crystal materials, optics, etc., to achieve the effects of large anisotropy and dielectric constant, wide nematic phase temperature, and high birefringence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] in formula middle
[0081] with R 1 The structure is -C 5 h 11 , R 2 The structure is -C 2 h 5 , R 3 The structure is -C 3 h 7 As an example (compound C10, referred to as PTNE503), the synthesis method of this liquid crystal monomer compound containing mononaphthalene series is introduced:
[0082] Step 1: Synthesis of Intermediate Z1:
[0083]
[0084] Add 59.25g (0.25mol) of 2-bromo-6-methoxynaphthalene and 413g of dichloroethane into a 1L three-necked flask, cool down to -10-0°C, and add 39.99g (0.3mol) of aluminum trichloride. 23.55g (0.3mol) of acetyl chloride was added dropwise, and the temperature of the system was raised to 0-10°C after the addition was completed. Insulation reaction 2h. Hydrolyzed, the organic phase was washed to neutrality. The solvent was removed to obtain 61.00 g of light yellow solid Z1 with a GC purity of 94% and a yield of 87.41%.
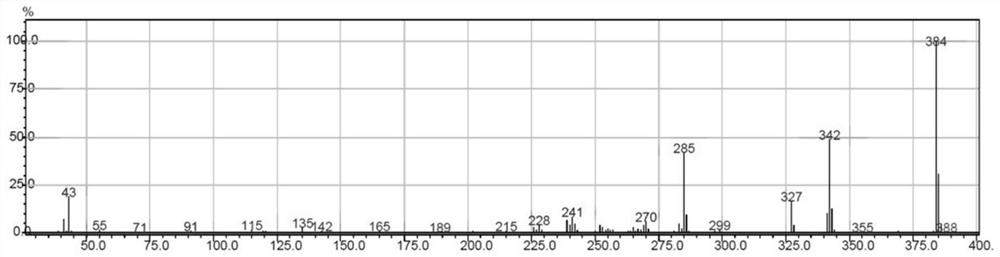
[0085] GC-MS: The theoretical value is 277.99, the actual value is 278.
[0086] Step 2:...
Embodiment 2
[0103] in formula middle
[0104] with R 1 The structure is -C 5 h 11 , R 2 The structure is -C 2 h 5 , R 3 The structure is -C 3 h 7 As an example (compound C09, referred to as PNE503), the synthesis method of this liquid crystal monomer compound containing mononaphthalene series is introduced:
[0105]
[0106] 40.33g (0.21mol) 4-pentylphenylboronic acid, 55.70g (0.19mol) intermediate Z3, 333.0g toluene, 29.02g (0.21mol) K 2 CO 3 and 87.0g of water were added to a 1L three-necked flask. Under nitrogen protection, 0.043 g (0.00019 mol) of palladium acetate and 0.099 g (0.00038 mol) of triphenylphosphine were added. Raise the temperature to 70-80°C and keep it warm for 3h. After the reaction was completed, the layers were allowed to stand, and the organic phase was washed with water until neutral, dried over anhydrous sodium sulfate, and filtered. The solvent was dried under reduced pressure to obtain a dark red liquid crude product. After recrystallization ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Clear point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com