Copolymerization solid electrolyte, preparation method thereof and solid polymer lithium battery
A solid electrolyte and solid polymer technology, applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, secondary batteries, etc., can solve the problems of low conductivity and difficulty in normal operation, achieve good conductivity and reduce the impact of battery performance , Improve the effect of cycle and energy density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of electrolyte precursor: the polymer monomer 1,3,5-trioxane (TXE) and the copolymer 2,2,2-trifluoro-N,N-dimethylacetamide (FDMA) were mixed by mass Mix at a ratio of 5:3 to dissolve the solid and mix evenly; then add 1mol / L lithium difluorooxalate borate (LiDFOB) and stir at 40°C for about 0.5h until completely dissolved, and finally obtain a fully mixed electrolyte precursor .
[0038]Preparation of solid electrolyte: The electrolyte precursor obtained above was heated at 55° C. for 10 h, so that LiDFOB initiated the polymerization reaction of TXE and copolymer to form a solid polymer, that is, to obtain a polymer solid electrolyte.
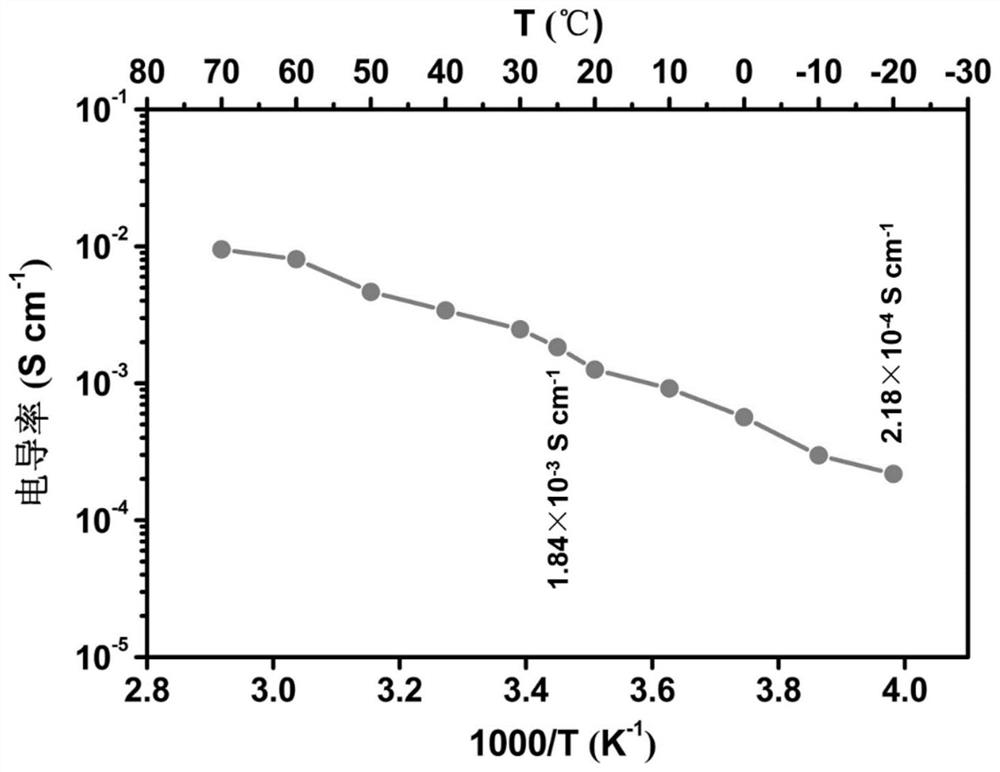
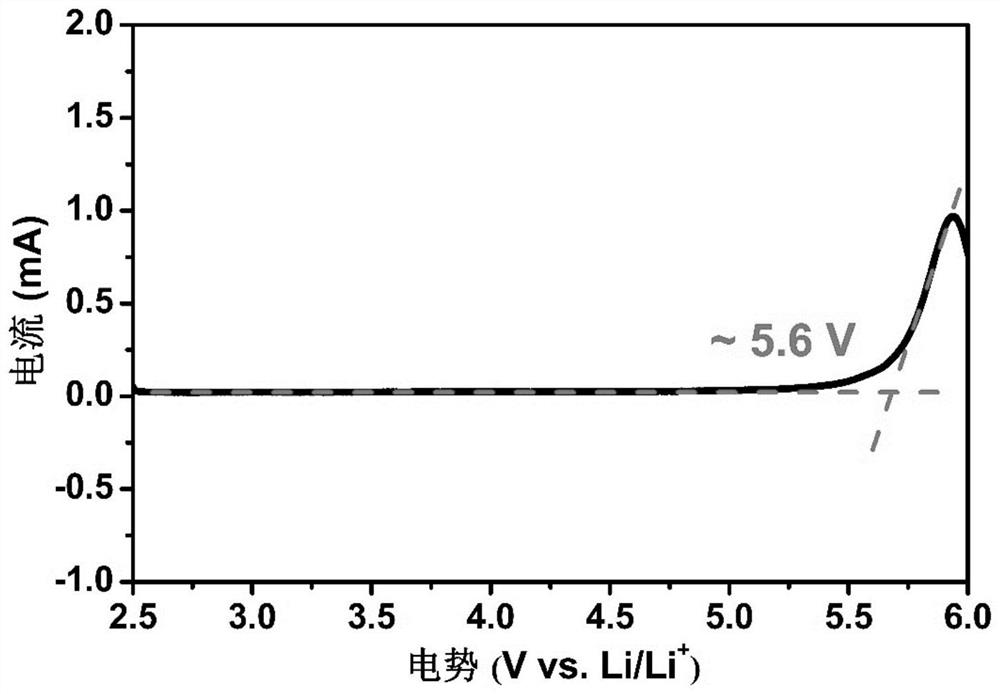
[0039] The above-mentioned solid electrolyte was solidified in a sealed electrolytic cell to test the ionic conductivity, and a 200 μm thick copper sheet was used as a double electrode to test the conductivity at various temperatures at -20 to 70°C. as attached figure 1 As shown, the conductivity increases with temperature,...
Embodiment 2
[0041] Preparation of electrolyte precursor: the polymer monomer 1,3,5-trioxane (TXE) and the copolymer 2,2,2-trifluoro-N,N-dimethylacetamide (FDMA) were mixed by mass Mix at a ratio of 5:3 to dissolve the solid and mix evenly; then add 1 mol / L lithium difluorooxalate borate (LiDFOB) and stir for about 15 minutes until completely dissolved, and finally a well-mixed electrolyte precursor is obtained.
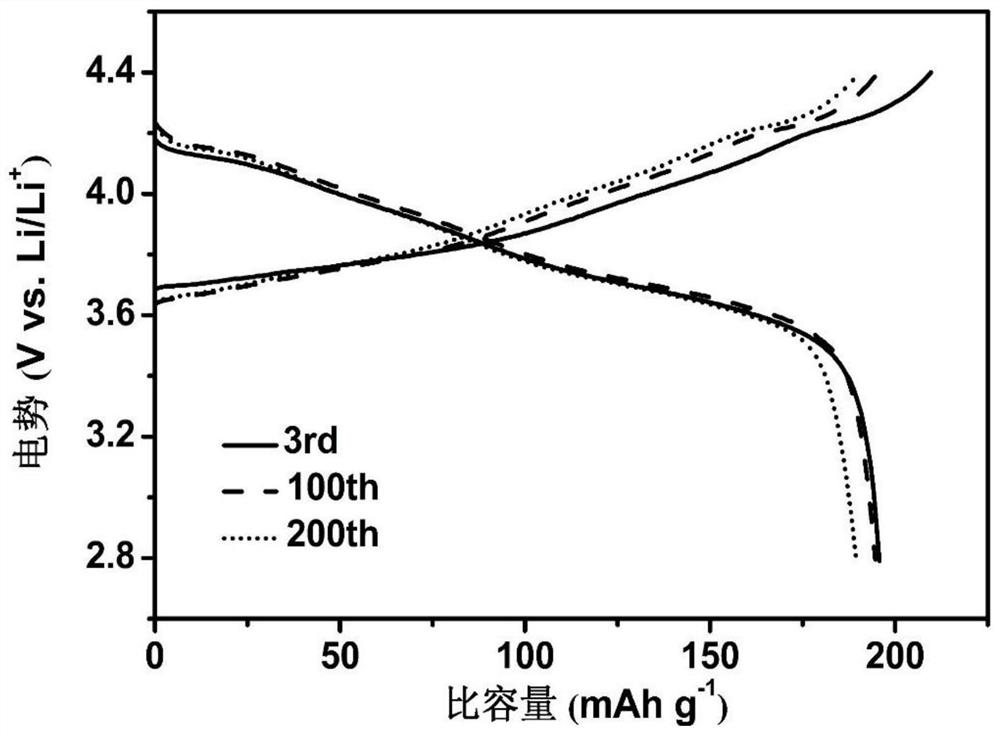
[0042] Preparation of solid electrolyte and assembly of solid-state battery: Take 10 μL of the obtained precursor and inject it between the positive and negative electrodes inside the battery, so that the liquid precursor to be polymerized can fully infiltrate the positive, negative and separator of the battery, and then complete the assembly of the battery; , the interpolymer is a fluorinated amide compound, and the positive electrode is LiNiCo 0.1 mn 0.1 o 2 (NCM811), the negative electrode is lithium metal, and the separator is Celagard2400. Finally, move the above-assemble...
Embodiment 3
[0047] Preparation of electrolyte precursor: the polymer monomer 1,3,5-trioxane (TXE) and the copolymer 2,2,2-trifluoro-N,N-dimethylacetamide (FDMA) were mixed by mass Mix at a ratio of 5:4 to dissolve the solid and mix evenly; then add 2mol / L lithium difluorooxalate borate (LiDFOB) and stir for about 20 minutes until completely dissolved, and finally a fully mixed electrolyte precursor is obtained.
[0048] Preparation of solid electrolyte and assembly of solid-state battery: Take 10 μL of the obtained precursor and inject it between the positive and negative electrodes inside the battery, so that the liquid precursor to be polymerized can fully infiltrate the positive, negative and separator of the battery, and then complete the assembly of the battery; , the interpolymer is a fluorinated amide compound, and the positive electrode is LiNiCo 0.1 mn 0.1 o 2 (NCM811), the negative electrode is lithium metal, and the separator is Celagard2400; finally, the assembled battery is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity at room temperature | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com