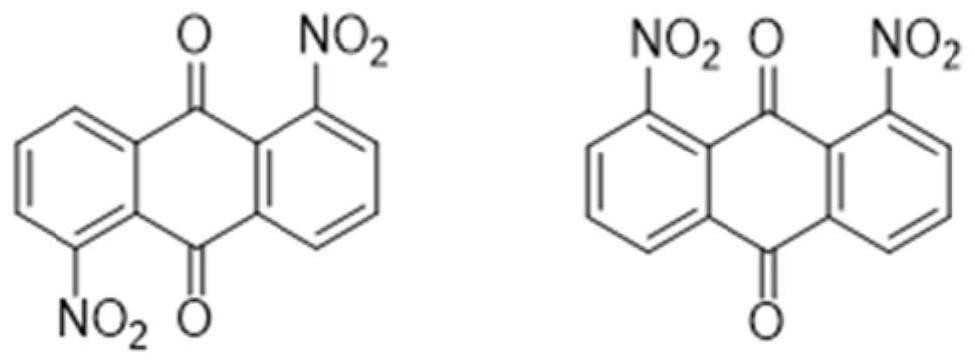
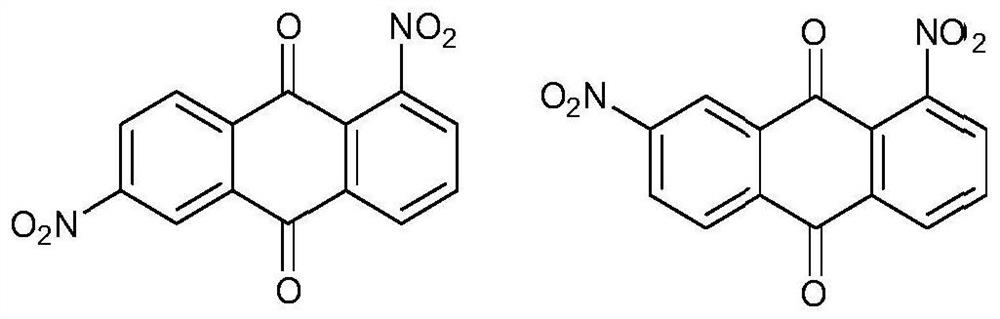
Phenoxylation process for preparing disperse blue 2BLN
A technology of phenoxylation and disperse blue, which is applied in the field of disperse blue preparation, can solve problems such as slowing down the production progress, achieve the effects of reducing by-products, facilitating drying, and avoiding caking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In this embodiment, a dispersed blue 2BLN phenoxylated product was prepared using the following method:
[0042] (1) The purification step is added to the product of sulfite after 醌 after a nitrification reaction, and the molar ratio of sodium sulfite and the sulfite primary nitrification product is 0.68: 1, and the system pH adjustment before joining sodium sulfite. 10.5;
[0043] (2) Potassium hydroxide and phenol are added to the phenoxylated reaction kettle, heated to 99 ° C, and heat it up to 145 ° C using the reaction heat, stirred at 145 ° C for 40 min, and the material is completely dissolved to 118 ° C The intermediate product obtained by adding step (1), allowing the molar ratio of phenol to the intermediate product to 2.65: 1, followed by warming to 147 ° C, and the insulation reaction is 4.5h;
[0044] (3) After the insulation reaction is completed in step (2), the mixed alkali is rapidly added to the reaction system under 95 ° C, stir mixed with 40 min, and the...
Embodiment 2
[0047] In this embodiment, a dispersed blue 2BLN phenoxylated product was prepared using the following method:
[0048] (1) The purification step is added to sodium sulfite in a product after hydrazine after a nitrification reaction, and the molar ratio of sodium sulfite and sulfite and the anthraquinone is 0.7: 1, and the system pH is adjusted before joining sodium sulfite. 12;
[0049] (2) Add potassium hydroxide and phenol, heated to 98 ° C, and heat it up to 143 ° C using the reaction heat, stirred at 143 ° C for 50 min, and cool down to 118 ° C after storage. The intermediate product obtained by adding step (1) so that the molar ratio of phenol and the intermediate product reaches 2.6: 1, followed by warming to 145 ° C, and the insulation reaction is 3.5 h;
[0050] (3) After the insulation reaction is completed in step (2), the mixed alkali is rapidly added to the reaction system under 97 ° C, and stir mixed with 35 min, and then the water of 95 ° C was diluted, and the mixt...
Embodiment 3
[0053] In this embodiment, a dispersed blue 2BLN phenoxylated product was prepared using the following method:
[0054] (1) The purification step is added to sodium sulfite in the product after after a nitrification reaction, and the molar ratio of sodium sulfite and the hydrazine primary nitrification product is 0.6: 1, and the system pH is adjusted before joining sodium sulfite. To 11;
[0055] (2) Add potassium hydroxide and phenol, heated to 95 ° C, and heat it up to 140 ° C using the reaction heat, stirred at 140 ° C for 45 min, and cool down to 120 ° C after storage. The intermediate product obtained by adding step (1) so that the molar ratio of phenol and the intermediate product reaches 2.7: 1, followed by warming to 142 ° C, and the reaction reaction is 6 h;
[0056] (3) After the insulation reaction is completed, the mixed alkali is rapidly added to the reaction system at 90 ° C, and the mixture is mixed at 90 ° C, and then the water of 90 ° C is diluted, and the dilutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com