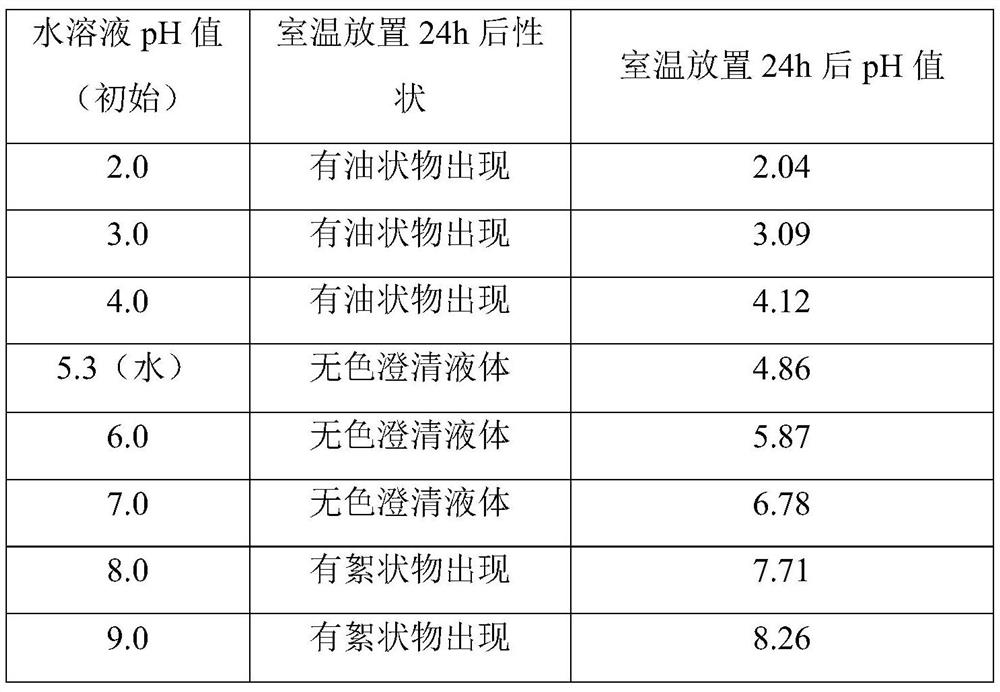
Vilanterol triphenylacetate inhalation solution and preparation method thereof
A technology of triphenylacetic acid and phenylacetic acid, applied in directions such as pharmaceutical formulation, liquid delivery, dispersion liquid delivery, etc., can solve problems such as inability to achieve drug delivery, and achieve the effects of alleviating liver and kidney damage, simple production process, and reduced drug concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
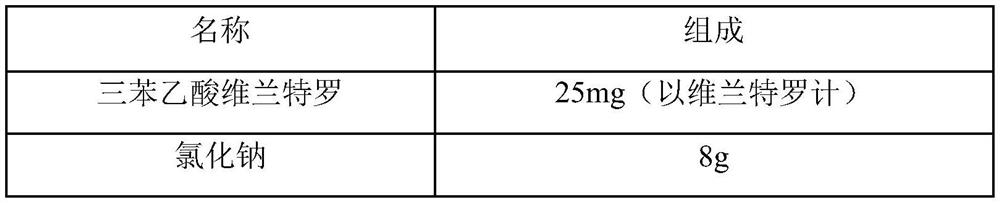
[0048] prescription composition
[0049] name composition vilanterol triphenylacetate 25mg (calculated as vilanterol) Sodium chloride 9g citric acid 0.05g sodium citrate 1g Add water for injection to 1000ml
[0050] Preparation:
[0051] Add 500ml of water for injection into the dispenser, control the water temperature to 75°C, add the raw and auxiliary materials to the above water for injection, stir until it is completely dissolved, add water for injection to the full amount, stir to mix evenly; use a 0.45μm filter membrane Perform primary filtration, 0.22μm filter membrane for fine filtration, all are aseptic filtration, sub-packaged and filled in ampoules.
Embodiment 2
[0053] prescription composition
[0054]
[0055]
[0056] Preparation:
[0057]Add 800ml of water for injection into the dispenser, control the water temperature to 75°C, add the raw and auxiliary materials to the above water for injection, stir until it is completely dissolved, then add water for injection to the full amount, stir to mix evenly; use a 0.45μm filter membrane Perform primary filtration, 0.22μm filter membrane for fine filtration, all are aseptic filtration, sub-packaged and filled in ampoules.
Embodiment 3
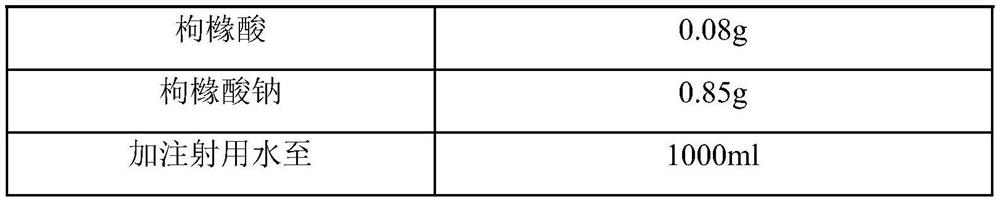
[0059] prescription composition
[0060] name composition vilanterol triphenylacetate 30mg (calculated as vilanterol) Sodium chloride 8g citric acid 0.05g sodium citrate 0.85g Add water for injection to 1000ml
[0061] Add 500ml of water for injection into the dispenser, control the water temperature at 80°C, add the raw and auxiliary materials to the above water for injection, stir until it is completely dissolved, add water for injection to the full amount, stir to mix evenly; use a 0.45μm filter membrane Perform primary filtration, 0.22μm filter membrane for fine filtration, all are aseptic filtration, sub-packaged and filled in ampoules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com