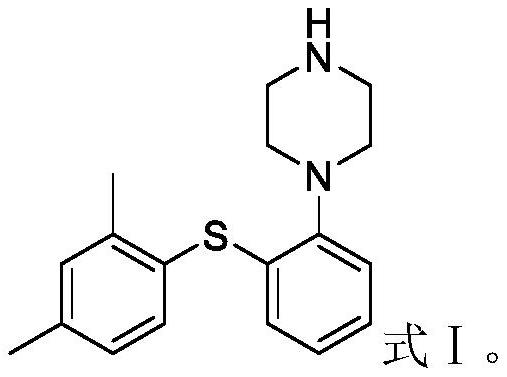
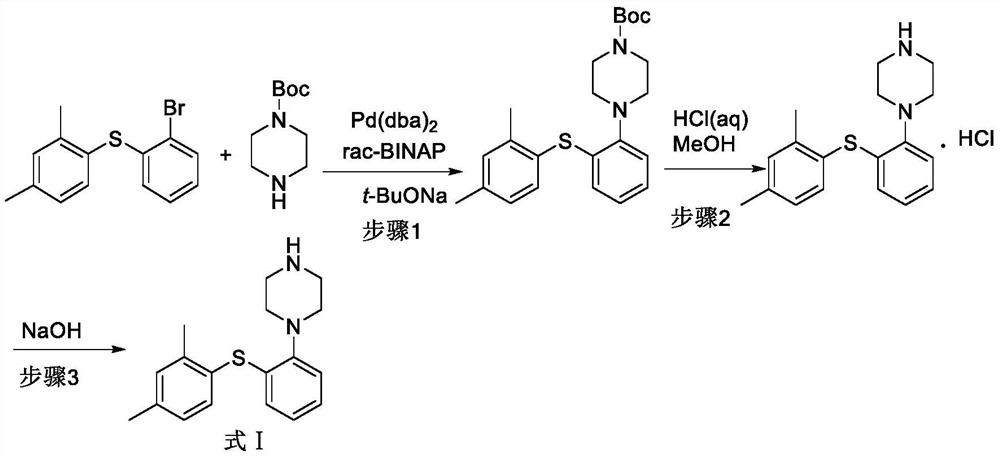
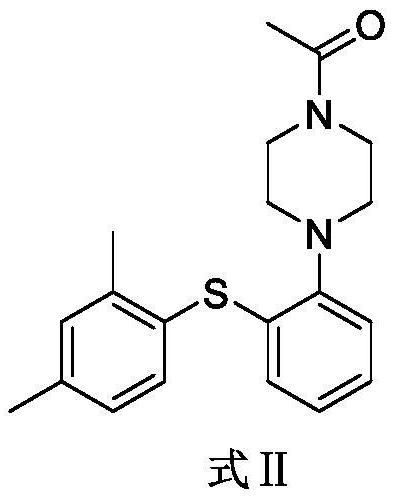
Preparation method of votioxetine
A technology of vortioxetine and propylene diamine, applied in the field of medicinal chemistry, can solve the problems of high price of resin palladium removing material, limited effect, difficult to clean and the like, and achieves the effect of good palladium removing effect and reduced operation steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Add 300mL of purified water and 75mL of 1,2-propanediamine (873.25mmol) into a 1000mL three-necked flask successively under stirring, control the temperature at 0-20°C, then slowly add 30.0g of vortioxetine hydrochloride (89.58mmol), Stir for 1 hour. Add 450 mL of ethyl acetate, stir for 2 hours, and let stand for liquid separation. The organic layer was washed successively with 300 mL of saline twice, and 300 mL of purified water twice. Dry over anhydrous sodium sulfate, filter with suction, collect the filtrate, add 1.5 g of activated carbon, stir and decolorize for 1 hour, and then filter with suction. The filtrate was collected and concentrated under reduced pressure until no liquid flowed out. Add 240mL of acetonitrile, heat at 70-80°C and stir to dissolve, add 1.5g of activated carbon, stir for 1 hour to decolorize, and suction filter while it is hot. The filtrate was cooled slowly to 10-15° C., stirred and crystallized for 4 hours, filtered with su...
Embodiment 2
[0032]
[0033] Add 300mL of purified water and 75mL of 1,2-propanediamine (873.25mmol) to a 1000mL three-neck flask successively under stirring, control the temperature at 0-20°C, and add 450mL of ethyl acetate. Add 30.0 g vortioxetine hydrochloride (89.58 mmol) slowly, stir for 3 hours, and let stand to separate the liquids. The organic layer was washed successively with 300 mL of saline twice, and 300 mL of purified water twice. Dry over anhydrous sodium sulfate, filter with suction, collect the filtrate, add 1.5 g of activated carbon, stir and decolorize for 1 hour, and then filter with suction. The filtrate was collected and concentrated under reduced pressure until no liquid flowed out. Add 240mL of acetonitrile, heat at 70-80°C and stir to dissolve, add 1.5g of activated carbon, stir for 1 hour to decolorize, and suction filter while it is hot. The filtrate was cooled slowly to 10-15° C., stirred and crystallized for 4 hours, filtered with suction, and the filter c...
Embodiment 3
[0035]
[0036] Add 200mL of 3moL / L sodium hydroxide solution into a 1000mL three-neck flask under stirring, control the temperature at 0-10°C, slowly add 20.0g of vortioxetine hydrochloride (59.72mmoL), after the addition is complete, continue to control the temperature and stir the reaction 4 After 1 hour, 300 mL of ethyl acetate was added and stirred for 1 hour. Stand still to separate the liquid, collect the organic phase, wash with 300 mL of saline × 2 times, and wash with 300 mL of purified water × 2 times. Dry over anhydrous sodium sulfate and filter. Collect the filtrate, add 1 g of activated carbon and stir for decolorization for 30 minutes, filter with suction, collect the filtrate, and concentrate under reduced pressure until there is no liquid. Add 160mL of acetonitrile, heat at 70-80°C and stir to dissolve, add 1g of activated carbon, stir for 1 hour to decolorize, and suction filter while it is hot. The filtrate was cooled slowly to 10-15° C., stirred and cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com