Mass spectrum-based epitope positioning method of in vitro diagnostic reagent antibody
A technology of in vitro diagnosis and positioning method, which is applied in measurement devices, material analysis by electromagnetic means, instruments, etc., can solve the problems of differences in binding sites, mutual recognition of quantitative results, and insufficient traceability process, so as to ensure accuracy. , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
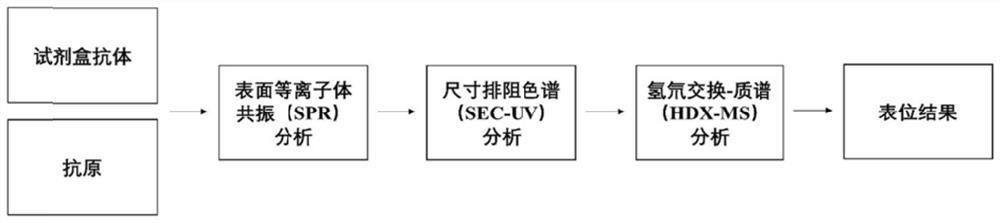
[0053] Such as figure 1 As shown, a mass spectrometry-based epitope mapping method for in vitro diagnostic reagent antibodies, the specific steps are:
[0054] 1. Antigen and kit antibody preparation
[0055] Select a kit for epitope analysis, prepare an antigen solution with a purity of more than 90% for the detection items of the kit, and determine its concentration. Antigens were stored in a -80°C freezer until relevant experiments.
[0056] Determine the composition type of the kit antibody (multi-antibody pairing or single antibody), collect all antibodies in the selected kit according to the type, and determine the type (multi-antibody / monoclonal antibody) and concentration of each antibody. Each antibody was stored in a -80°C freezer until the relevant experiment.
[0057] 2. Antigen purity and aggregation state analysis
[0058] 2.1 Antigen purity analysis
[0059] The epitope mapping method based on hydrogen-deuterium exchange mass spectrometry usually requires t...
Embodiment 2
[0119] The method of Example 1 was used to locate the epitope of the antibody of the whole process C-reactive protein assay kit of a domestic company, and the specific experiment was as follows:
[0120] 1. Preparation of C-reactive protein (CRP) and a company's whole-process C-reactive protein assay kit antibody
[0121] A natural CRP solution (Beijing Deoping Biotechnology Co., Ltd.) with a purity of 95% was prepared at a concentration of 2.05 mg / mL. Jiuqiang kit contains a polyclonal antibody with a concentration of 13.66mg / mL. The above samples were stored in a -80 °C freezer before the experiment.
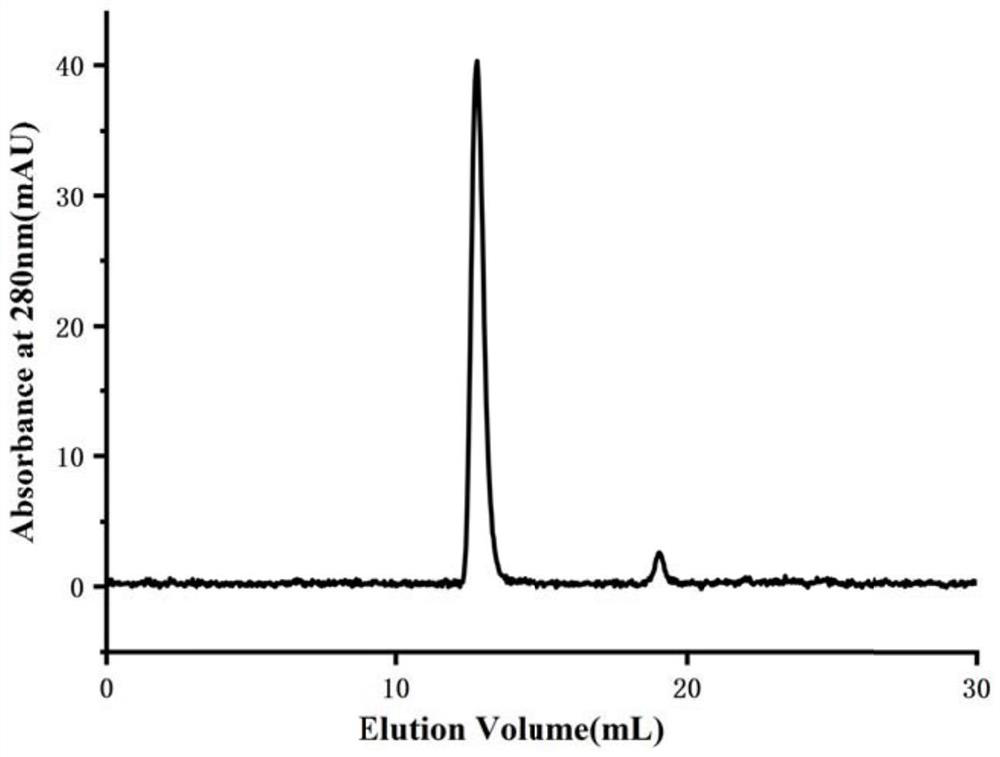
[0122] 2. Antigen purity and aggregation state analysis
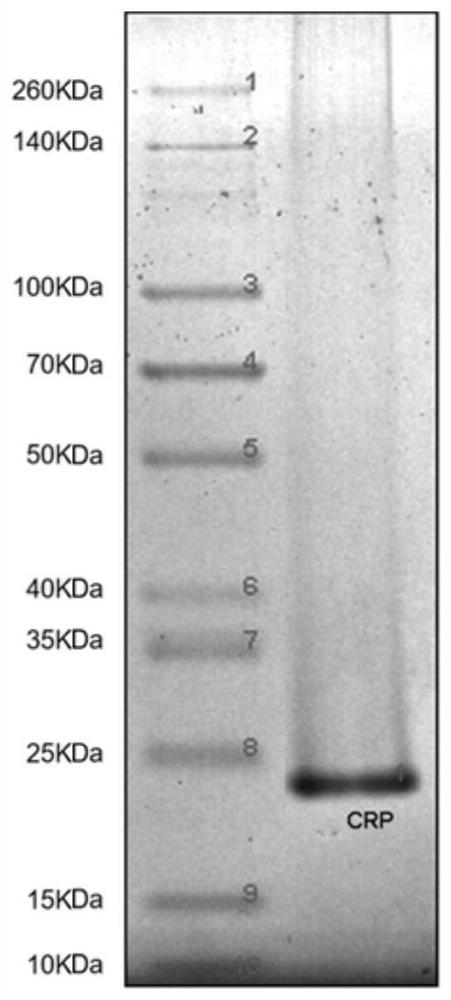
[0123] 2.1 Antigen purity analysis (such as figure 2 )
[0124] SDS-page gel electrophoresis was used to verify the purity of CRP.
[0125] The specific operation method of SDS-page is as follows:
[0126] 1) Fix the glass tank, check for leaks with deionized water, prepare a separation gel with a suitable concen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com